Introduction
The wettability alteration mechanisms during LS water flooding are less understood than other EOR techniques. The mechanisms of LS water flooding are still a topic of debate. The incremental oil recovery from sandstone using LS water include several proposed mechanisms: mineral dissolution[1], multi-component ion exchange[2], double-layer expansion[3], desorption of organic material from clay surface[4], reduction in interfacial tension[5], and fines migration[6]. The presence of clay in the reservoir[2,3,4,5,6] has a significant impact on oil recovery. The chemical composition of the injected water is another major factor affecting additional oil production[4,7-8]. However, there is no consensus on the dominant mechanism of enhancing recovery in sandstone reservoirs[4,8-9]. This may be because several simultaneous processes contribute to the overall process. To our knowledge, no systematic experimental and numerical studies have been carried out that consider the most important water-rock interactions in sandstones simultaneously.
Reservoir chemical heterogeneity may also play a role. Experimental and field scale projects indicate that incremental oil recovery by LS water flooding varies significantly case- by-case in both carbonates[10,11] and sandstones[5,6,7,8,9,10,11,12]. Minerals in natural porous media are typically distributed unevenly with random spatial patterns, ranging from uniform distribution to clustered minerals[13,14]. On one hand, physical heterogeneity changes flow fields and therefore the spatial distribution of ions[15]. On the other hand, chemical heterogeneity significantly changes dissolution rate[16,17] and adsorption/desorption[18]. Combination of physical and chemical heterogeneity can significantly impact water-rock interaction and wettability alteration. However, the effect of spatial distribution of reservoir physical and chemical properties on water-rock interaction and wettability alteration during LS water flooding has not been fully considered.
In this study, we quantify the effect of mineral composition and water chemical properties on the water-rock reaction and wettability alteration. The mechanisms of LS waterflooding in sandstone with and without clay under different temperatures are examined by using two chromatographic columns containing free-clay quartz and quartz with clay, and the effect of clay on recovery enhancement is analyzed.
1. Methodology
1.1. Column packing
A chromatography column was packed with minerals to imitate sandstone cores. Using such a column allows us to control the mineralogy of the porous media. The sandstone core without clay was made up of pure quartz, while the sandstone core with clay was composed of 5% illite, 5% kaolinite and 90% quartz. The cores are all 6.3 cm long and 1.5 cm in diameter. In order to avoid air bubbles in the column, which can change the hydraulic conductivity, the columns were packed using wet packing method[19]. The connections to the column ports were supplied with a fine filter to prevent mineral grains from moving out of the column.
1.2. Water flooding
The column was flushed with HS water and then aged for a week at 70 °C with the same HS water containing 10 mmole sodium acetate. This was done to maximize adsorption of carboxylic material to the surface of the rock so as to simulate bonding of carboxylic material to reservoir rock. The importance of the carboxylic acid is shown in Fig. 1. The system was then flooded with HS water until the pH of the fluid out of the column stabilized. Water samples were collected at the column outlet for later analysis of ionic concentrations. After each experiment, the column was aged in the same condition for a week to restore the sample to the initial state. The whole experiment was done inside an oven set to the temperature of interest.
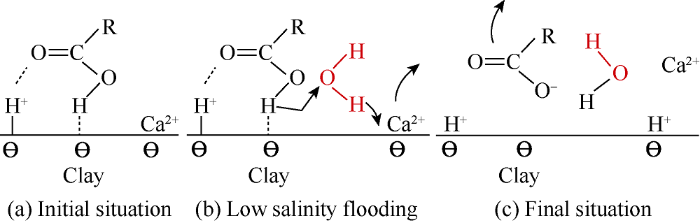
Fig. 1.
Carboxylic acid on the clay surface during LS water flooding[4].
1.3. Chemical analysis
The Ca+2 concentration of produced fluid was measured using (2000D ICP-OES). The acetate concentration was measured using a Dionex DX-120 ion chromatography setup.
1.4. Preparation of brines
HS brine was prepared by dissolving reagent CaCl2 and NaCl in Deionized water, and LS water was prepared by dissolving reagent NaCl only in deionized water. Compositions of the brines are listed in Table 1.
Table 1 Composition of HS and LS brines (mg/l).
| Element | HS | LS |
|---|---|---|
| Na+ | 35 000 | 350 |
| Cl- | 60 000 | 600 |
| Ca2+ | 4 500 | 0 |
| Acetate (aging) | 820 | 0 |
| TDS | 98.32 | 1.182 |
| Salinity | ~100 000 | ~1 000 |
1.5. Oil recovery test
Another column was flushed with HS water and saturated with oil and aged for a week at 70 °C. HS water and then LS water were injected into the column, and oil recovery was measured from the produced fluid.
1.6. Wettability measurement
We utilized the new method described in our work[20] to measure wettability in this study.
2. Results and discussion
2.1. Sand column without clay
2.1.1. Chemical analysis results of the effluent
This column containing quartz only, no clay was presented in this column. The permeability of Sand Column is 602×10-3 μm2 and the porosity measured by HS water is 33.64 %. At 25 °C, the pH was 7.26 when flooded with HS water. Upon switching to LS water, the pH rose to pH 9.77. When the injected water was transferred to the original HS water, the pH fell back to 7.29. There was about a 2.5 pH difference between HS water effluent pH and LS water effluent pH.
At 70 °C, the pH for the HS water effluent was 7.28. The water then switched to the LS water and pH for the LS water effluent was 9.72. After that, switching the flooding to the original HS water stabilized the pH back to its original value. An important variance ~2.44 in pH between HS water and LS water effluents was observed.
The pH trend is about the same at 90 °C. The pH stabilized at 7.23 while flooding the column by HS water, after switching to LS water the pH increased directly and stabilized at 9.32. The injected fluid switched again to the original HS water and pH return to its original value.
At 120 °C, the pH initially was 7.15 when flooded Column 1 by HS water. The pH suddenly rose and stabilized at 9.20 pH unit when switching the flooding to LS water. The injected fluid then switched again to the same HS water, and the pH fluctuated until fell again to its original value.
Traditionally, it is believed that the rise of pH value during LS water flooding is caused by the replacement of Ca2+ adsorbed on the surface of clay by H+ (ion exchange). Flooding the free-clay column with LS water, the same trend was observed (pH upward shift). We attributed that to the exchange of Ca2+ and Na+ absorbed on negatively charged site of quartz by H+.
As can be noticed from Table 1 that LS water contains no Ca2+, but the collected fluid after LS water flooding contains Ca2+ (Fig. 2), verifying the desorption of Ca2+ from quartz surface. The desorption of Ca2+ is the result of ion exchange between Ca2+ and H+ and Na+ on the surface of negatively charged quartz.
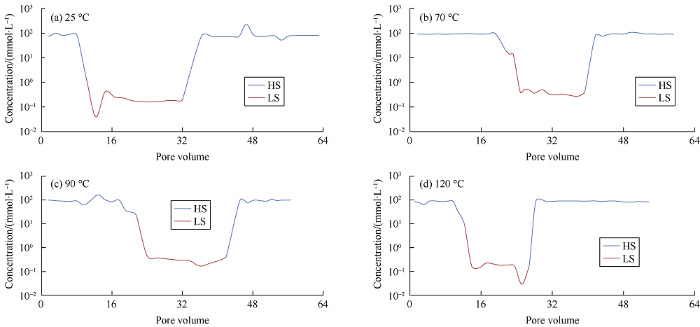
Fig. 2.
Effluent concentrations of Ca2+ from Sand Column at 25, 70, 90, and 120 °C.
The core was aged in HS water containing 10 mmole sodium acetate for a week at 70 °C, then it was flooded by HS water and then LS water at different temperatures, the concentration of acetate in the produced fluid from the flooding was measured, and its variations under different temperatures are shown in Fig. 3. It needs to be noted that the injected fluid volume under all temperatures were 40 times of pore volume. It can be seen from the Fig. 3 that when the sandstone core without clay is flooded by HS and LS water, the desorption of carboxylic materials has no correlation with the rise of temperature. As can be noticed, the detachment of CH3COO- was higher at 25 °C than at 70 °C, but that at 70 °C was lower than that at 90 °C, and that at 120 °C is the lowest.
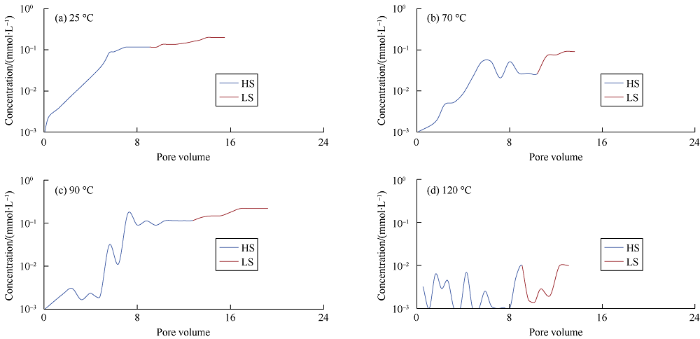
Fig. 3.
Concentrations of RCOO- in first HS and LS water effluent at 25, 70, 90, and 120 °C.
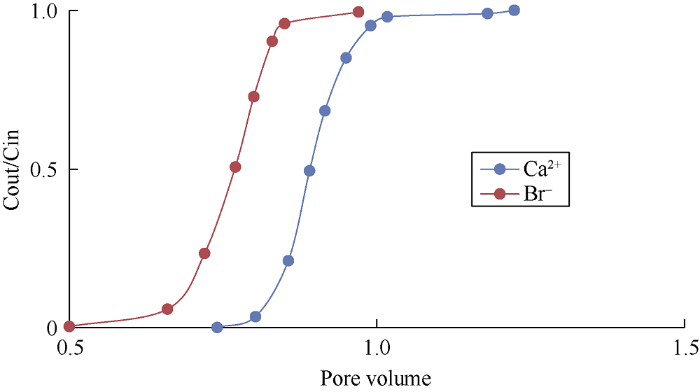
Fig. 4.
Concentrations (C) of Ca2+ and Br- while injecting HS water.
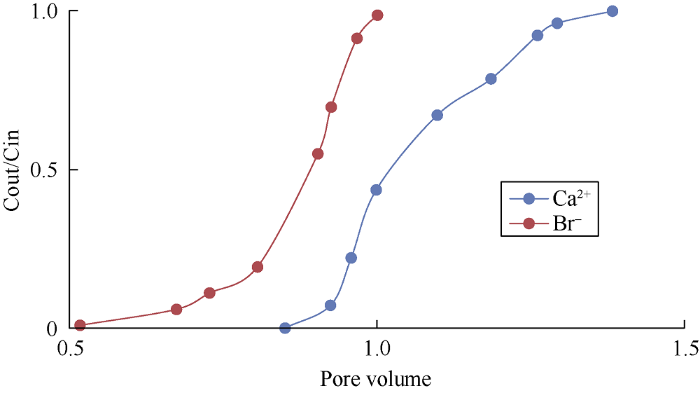
Fig. 5.
Concentrations of Ca2+ and Br- while injecting LS water.
In conclusion, because quartz surface and carboxylic materials are both negatively charged, they are repelled from each other. Indeed, the carboxylic material bonded with divalent cation Ca2+ [-COOCa+] [>Si-O2-] in form of electrostatic bridging with quartz surface. When LS water invades into the porous media, ion exchange takes place, then organic complexes are removed and replaced with uncomplexed cations, providing a more water wet environment and in turn enhancing recovery. At 25 °C, 70 °C, 90 °C, and 120 °C, the differences of pH values of the effluents from flooding by HS water and LS water are 2.5, 2.44, 2.09, and 2.04 respectively. It can be seen with the rise of temperature the difference of pH becomes smaller. It can be seen from Fig. 2 that when the core without clay was flooded with LS water at 25 °C, 70 °C, 90 °C, and 120 °C, about 0.32, 0.37, 0.32, and 0.16 mmole of Ca2+ desorbed from the quartz surface respectively. When the HS water was changed to LS water, a large amount of CH3COO- was detached from quartz surface. It can be seen from Fig. 3 that the acetate released was 10.60%, 8.35%, and 3.80% at 25, 70, and 90 °C, respectively, while the concentra-tion curve of CH3COO- was not stable at 120 °C. The observed results of Ca2+ desorption and CH3COO- detachment was in line with our expectations, verifying LS water flooding can facilitate the detachment of carboxylic acid from sandstone without clay, and thus improve recovery.
2.1.2. Oil Recovery Test
The sandstone core without clay was initially flooded by HS water at 25 °C until no additional oil recovery was observed the oil recovery factor was 41% OOIP. The injected fluid then was switched to LS water and the oil recovery increased by 4% to 45.0% OOIP. At 70 °C, 90 °C, and 120 °C, the incremental oil recovery was 2%, 1.5% and 1% OOIP respectively.
2.1.3. Wettability alteration
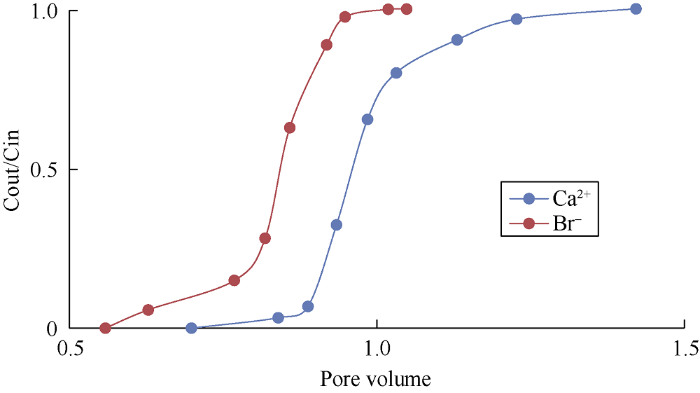
Figs. 4 and 5 show the wettability alteration when HS and LS water were used to flood the sand column without clay, the areas (Ao) between Ca2+ and Br- curves were 0.14 and 0.185 respectively. It can be known from Fig. 6 that when the core is totally water-wet, the area between Ca2+ and Br- curves (AH) is 0.22 (Fully saturated with heptane). The wettability index of the porous media can be calculated by dividing Ao by AH. The wettability index ranges from 0 for strongly oil-wet to 1 for strongly water-wet and 0.5 for intermediate wettability[20]. Wettability index of the sandstone core without clay when flooded by HS water was 0.63, while it was 0.84 when flooded by LS water, meaning that the wettability of the core becomes more water-wet when flooded with LS water.
Fig. 6.
Concentrations of Ca2+ and Br- for the water-wet core.
2.2. Sand column with clay
This column contains 5% illite + 5% kaolinite + 90% quartz. The column was packed and prepared the same way as the sand column. When flooded with HS water at 25 °C, the core had a recovery factor of 41.85% OOIP and pH value of the produced fluid from the core was 7.14. At the same temperature, flooding the column with LS water, the recovery factor increased by 3.7% OOIP%, for the core without clay, the recovery increment was 4.0%; and the pH of the produced fluid was 8.90. The core was flooded with LS water at temperatures of 70 °C, 90 °C and 120 successively, and the incremental oil recoveries were 2.45%, 1%, and 0.70%, and the pH of produced fluids were 8.78, 8.10 and 8.00 respectively. It can be seen that the recovery factors of core without clay and core with clay flooded by LS water differ little, suggesting the existence of clay has little effect on recovery factor.
The experimental observations of Tang and Morrow[6] for LS water flooding set out conditions for how LS water works. The conditions were: (1) the crude oil must contain acid and base numbers and (2) sandstone should contain clay such as illite and kaolinite. After several years, McGuire[21] and Lager and Webb[2] added another condition, which was that divalent cations must be present in the FW. In this study, we examined the role of clay during LS water and the results revealed that the clay role is weak. This study provides insights into the mechanisms that control LS EOR.
3. Conclusions
When the core without clay was flooded with LS water, the amount of CH3COO- and Ca2+ released were large, indicating ion exchange on the surface of quartz, and LS water can facilitate the detachment of carboxylate from the core, and thus improving oil recovery. In the flooding of sandstone with LS water, clay has little effect on recovery, even with no clay in the sandstone, the flooding can improve recovery factor.
The approach of this work is that it allows us to isolate the interplay between ion exchange, pH, and carboxylate release, which should allow us to better decode the chemical mechanisms that control LS water EOR flooding in sandstones. The following conclusions were established:
(1) Clays are not essential for sandstone during LS water flooding.
(2) Oil recovery increased during LS water flooding even though there was no clay in the porous media.
(3) Ca2+ desorption and acetate released from quartz were as high as when clay exists, meaning that an ion exchange and carboxylic detachment occurred on the quartz surface.
Reference
Evaluation of low-salinity enhanced oil recovery effects in sandstone: Effects of the temperature and pH gradient
DOI:10.1021/ef300162n URL [Cited within: 1]
Low salinity oil recovery: An experimental investigation
Novel waterflooding strategy by manipulation of injection brine composition
Chemical mechanism of low salinity water flooding in sandstone reservoirs
Low salinity oil recovery: An exciting new EOR opportunity for Alaska’s North Slope
Influence of brine composition and fines migration on crude oil brine rock interactions and oil recovery
Smart water as wettability modifier in carbonate and sandstone: A discussion of similarities/differences in the chemical mechanisms
DOI:10.1021/ef900185q URL [Cited within: 2]
Chemical verification of the EOR mechanism by using low saline/smart water in sandstone
DOI:10.1021/ef200215y URL [Cited within: 3]
Investigation of wettability alteration and oil-recovery improvement by low-salinity water in sandstone rock
DOI:10.2118/146322-PA URL [Cited within: 2]
Wettability alteration and improved oil recovery by spontaneous imbibition of seawater into chalk: Impact of the potential determining ions Ca 2+, Mg 2+, and SO4 2-
Improved/ enhanced oil recovery from carbonate reservoirs by tuning injection water salinity and ionic content
LoSoI TM enhanced oil recovery: Evidence of enhanced oil recovery at the reservoir scale
Geochemical heterogeneity in a sand and gravel aquifer effect of sediment mineralogy and particle size on the sorption of chlorobenzenes
DOI:10.1016/0169-7722(92)90049-K URL [Cited within: 1]
When good statistical models of aquifer heterogeneity go bad: A comparison of flow, dispersion, and mass transfer in connected and multivariate Gaussian hydraulic conductivity fields
Solute transport in low-heterogeneity sand boxes: The role of correlation length and permeability variance
DOI:10.1002/2013WR014654 URL [Cited within: 1]
Magnesite dissolution rates at different spatial scales: The role of mineral spatial distribution and flow velocity
DOI:10.1016/j.gca.2013.01.010 URL [Cited within: 1]
Spatial zonation limits magnesite dissolution in porous media
DOI:10.1016/j.gca.2013.10.051 URL [Cited within: 1]
Illite spatial distribution patterns dictate Cr(VI) sorption macrocapacity and macrokinetics
Hydrologic flow controls on biologic iron (Ⅲ) reduction in natural sediments
New wettability method for sandstone using high-salinity/low- salinity water flooding at residual oil saturation. SPE 190464-
Low salinity oil recovery: An exciting new EOR opportunity for Alaska’s North Slope