Introduction
Fluid flowing in the rock mainly depends on the pore system. The presence of highly soluble carbonate minerals would result in changes of pore geometry and petrophysical properties in carbonate reservoirs under fluid-rock interactions[1,2]. These interactions at pore scale can be used to improve the performance of carbonate reservoirs[3,4,5,6,7] and to evaluate the reservoir integrity for long-term Carbon Capture and Storage (CCS)[8,9,10,11]. Knox et al.[3] considered that the performance of acidizing was affected by multiple factors, including acid penetration, acid density, fracture flow capacity, temperature, acid concentration, fluid density and viscosity. Mcleod et al.[4] attributed the success of acidizing to the evaluation of production history, as well as the design for acid damaged perforations such as selection of solvents and acid components. McDuff et al.[5] used a new 3D visualization method to evaluate the performance of acidizing on carbonate reservoirs.
Several researchers reported that pore structure changes caused by dissolution was controlled by multiple factors such as fluid temperature, pressure, pH, porosity, permeability and crystal size[12,13,14,15,16,17,18,19,20]. However, there are few studies on the change of pore network attribute induced by dissolution reported.
The X-ray micro tomography (X-Ray micro CT) technique is now commonly used to study pore systems in reservoir[21,22,23]. The 3D pore network information extracted via several approaches such as segmentation binarization or skeletonization can give us an understanding on fluid flowing within complex porous media, through working out attributes such as porosity distribution, pore radius, pore throat radius, pore throat length and coordination number[24,25,26,27,28,29,30].
X-ray micro CT can be also used to evaluate dissolution induced pore changes at various conditions (different temperatures and pressures). Luquot et al.[1] analyzed the impact of CO2-rich brine on limestone reservoir properties and concluded that porosity and permeability changes were controlled by inlet fluid disequilibrium and the initial reaction rate. Noiriel et al.[31] evaluated the 3D changes of fractures in limestone at room temperature and noticed that there was no preferential flow pathways formed and the presence of any silicates in carbonate rocks led to heterogeneous dissolution at micro-scale. Menke et al.[11] investigated the dynamic evolution of pores in carbonate rocks saturated with CO2 brine at 50 °C and 10 MPa and concluded that the ratio of surface area to volume and porosity increased. Rötting et al.[32] found that significant dissolution only occurred in certain pore diameter at specific conditions. Even though, these researches have given important knowledge on pore system modifications at different pressure and temperature conditions, the impact of dissolution on carbonate pore systems still needs further study[33,34,35,36,37]. Therefore, the objective of this study is to find out the effect of dissolution on pore network in carbonate rocks at various well conditions and temperatures.
1. Materials and methods
1.1. Dissolution experiment
The dissolution experiments of two types of carbonate rocks, mudstone (type 1 rock) and grainstone (type 2 rock), were performed in a closed bath reactor system[38,39]. The pH, concentration of HCl and rotation rate of stirrer were maintained constant at 1.2, 0.1 mol/L, 12.56 rad/s respectively during the experiments. The dissolution experiment was conducted at 25 °C, 50 °C and 75 °C for 100 min. Aliquots of samples were collected every 10 min and the calcium concentration in each aliquot was tested using Plasma-Optical Emission Spectrometry (Perkinelmer, Optima 8300 ICP-EOS Spectrometer) to figure out the Ca2+ concentration released by the carbonate rock sample. Variation of Ca2+ concentration with time was analyzed to find out the dissolution rate law and characteristics of dissolution kinetic.
1.2. X-Ray Micro CT imaging
Each sample was scanned with X-Ray CT System (inspeXio SMX-255CT) at 160 kV and 100 µA. After scanning, the images were processed and then pore networks were extracted using VGStudio Max 2.1, ProAnalyzer, Fiji ImageJ and Avizo Fire software. In order to evaluate the impact of dissolution on fluid flow properties of the samples, Avizo Fire software was used to simulate the permeability before and after dissolution. These simulations were performed following Darcy’s law, under the following conditions: inlet and outlet pressures of 2.75×106 Pa and 0.10×106 Pa respectively, and the viscosity of 1.96×10-5 Pa·s (helium viscosity at room temperature).
1.3. Petrophysical analysis
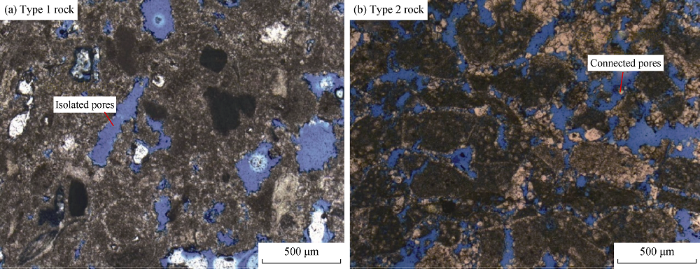
Thin section observation of both rock types shows that they differ widely in pore characteristics. Type 1 rock has pores that are restricted and isolated within the general fabric of the rock (Fig. 1a). Type 2 rock has pores that are interconnected and distributed throughout the rock fabric (Fig. 1b).
Fig. 1.
Fig. 1.
Pore characteristics of two types of carbonate rocks.
Comparing the two types of rock shows type 1 rock has a porosity of 20% and permeability of 1.39×10-3 μm2; type 2 rock has a porosity of 36% and permeability of 1 063.38×10-3 μm2. From XRD analysis, type 2 rock is composed of 96.88% of calcite and 3.12% of dolomite whereas type 1 rock is composed of 87% of calcite and 13% of dolomite. In terms of the mineralogical composition, type 1 rock contains more stable minerals than type 2 rock. This implies that type 2 rock would dissolve faster than type 1 rock.
According to the different dissolution temperatures (25, 50 and 75 °C), each rock type was divided in three portions. The type 1 rock portions were coded as M1, M2, M3 and the type 2 rock portions were B1, B2 and B3.
2. Results and discussions
2.1. Dissolution analysis
2.1.1. Relationship of calcium ion concentration and time
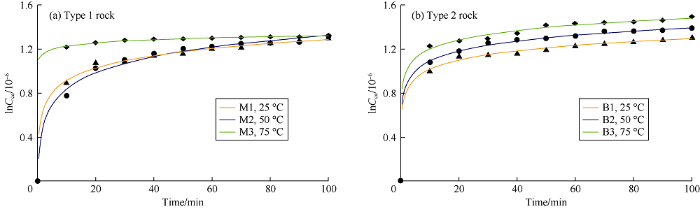
As can be seen from Fig. 2, the released Ca2+ increases rapidly in the first 20 min at the initial stage of dissolution, and the increase of Ca2+ concentration gradually slows down with time. The second phase of dissolution is stable, which is related to the gradual decrease of released Ca2+. The temporal variation of released Ca2+ is described by the following general equation (Eq. 1) and obtained from the best fit model of Ca2+ concentration and time curve at 25, 50 and 75 °C.
where $x=\lg t$
Fig. 2.
Fig. 2.
Relationship between Ca2+ concentration released from two types of carbonate rocks and time at different temperatures.
The generated model follows the first-order reaction characteristics and can be expressed by linear equation. The new model is defined by two constants, the rate constant k and the initial Ca2+ concentration Cca,0. The time and Ca2+ concentration show good correlation, with R2 of 0.9. The dissolution experiments show carbonate dissolution is stronger at higher temperature (Fig. 2).
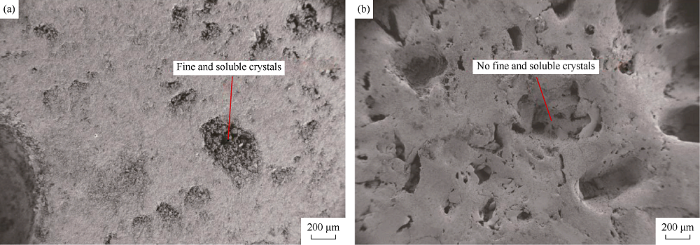
The fast increase of Ca2+ concentration initially can be attributed to the presence of highly soluble, fine and unconsolidated carbonate crystals or grains and unstable and highly reactive minerals at the rock surface (Fig. 3). Scanning electron microscopic image of Fig. 3a shows calcite mineral on type 1 rock surface before dissolution. Fig. 3b shows type 1 rock after dissolution, in which most of the fine and soluble particles have been dissolved, leaving a more porous and smoother rock surface. Once these unstable carbonate components are dissolved, the release of Ca2+ generally decreases and the dissolution remains constant. This could possibly be due to the gradual saturation of the aqueous solution, or reduction of highly reactive surface minerals as the experimental conditions remain unchanged (pH remains constant).
Fig. 3.
Fig. 3.
SEM images before and after dissolution of type 1 rock.
2.1.2. Dissolution kinetic models
The instantaneous dissolution rate model is a function of rate constant and the released Ca2+ concentration, and can be written as the following equation (2).
The dissolution kinetic model has characteristic of first order reaction. Based on this model, dissolution rate depends on initial concentration of Ca2+, the rate constant and laboratory conditions (Table 1). Type 1 sample dissolved slowest at 25 °C and type 2 sample dissolved fastest at 75 °C. The comparison of dissolution rates of the 2 types of carbonate rock shows that type 1 dissolves slower than the type 2 at any given temperature. This is attributed to the type 1 (13% of dolomite) contains more stable carbonate minerals than type 2 (3% of dolomite). As the experimental conditions of dissolution were identical, the variation of rate constant between the samples is attributed to the heterogeneities of rocks. These heterogeneities include chemical element composition and petrophysical characteristics (porosity and permeability).
Table 1 Dissolution kinetics parameters of 2 kinds of carbonate rocks at 25, 50 and 75 °C.
| Tempera- ture/°C | Type 1 rock | Type 2 rock | ||||
|---|---|---|---|---|---|---|
| Cca,0/10-6 | k/(10-6·min-1) | DR*mean/(10-6·min-1) | Cca,0/10-6 | k/(10-6·min-1) | DR*mean/(10-6·min-1) | |
| 25 | 1.711 | 0.163 | 0.198 | 2.085 | 0.122 | 0.202 |
| 50 | 1.417 | 0.212 | 0.206 | 2.201 | 0.131 | 0.244 |
| 75 | 3.095 | 0.041 | 0.211 | 2.503 | 0.122 | 0.312 |
2.2. Pore network system induced by dissolution
Table 2 summarizes the pore network system variations at different temperatures. The pore networks are characterized by several attributes, including porosity, pore size distribution, number of pores, pore throat radius, and pore throat length and coordination number. The permeability is also affected by dissolution as a consequence of pore network variation.
Table 2 Pore network model variation induced by dissolution at various temperatures.
| Rock type | Sample and temperature | Experimental condition | Measured porosity/% | Pore number | Pore size/mm | Pore throat number | Average pore throat radius/mm | Average pore throat length/mm | Coordination number | Absolute permeability/ 10-3 μm2 |
|---|---|---|---|---|---|---|---|---|---|---|
| Type 1 rock | M1, 25 °C | Before dissolution | 1.70 | 801 | 0.18 | 411 | 0.022 | 0.27 | 4.2 | 13.04 |
| After dissolution | 5.20 | 564 | 0.24 | 291 | 0.030 | 0.37 | 5.3 | 50.00 | ||
| M2, 50 °C | Before dissolution | 0.11 | 25 | 0.21 | 9 | 0.049 | 0.53 | 1.0 | 4.16 | |
| After dissolution | 0.43 | 13 | 0.32 | 6 | 0.065 | 0.65 | 2.9 | 17.75 | ||
| M3, 75 °C | Before dissolution | 0.23 | 794 | 0.24 | 313 | 0.035 | 0.38 | 1.2 | 2.50 | |
| After dissolution | 1.06 | 274 | 0.26 | 88 | 0.050 | 0.52 | 2.2 | 9.45 | ||
| Type 2 rock | B1, 25 °C | Before dissolution | 8.07 | 4 139 | 0.10 | 792 | 0.026 | 0.23 | 3.0 | 751.53 |
| After dissolution | 12.70 | 1 637 | 0.18 | 515 | 0.036 | 0.33 | 4.4 | 950.00 | ||
| B2, 50 °C | Before dissolution | 6.80 | 1 604 | 0.19 | 348 | 0.052 | 0.51 | 2.7 | 843.40 | |
| After dissolution | 10.80 | 875 | 0.30 | 195 | 0.055 | 0.56 | 4.0 | 2 358.30 | ||
| B3, 75 °C | Before dissolution | 8.01 | 1 588 | 0.12 | 691 | 0.015 | 0.18 | 4.9 | 1 638.50 | |
| After dissolution | 22.34 | 1 035 | 0.23 | 321 | 0.053 | 0.53 | 7.9 | 5 556.50 |
2.2.1. Porosity variations
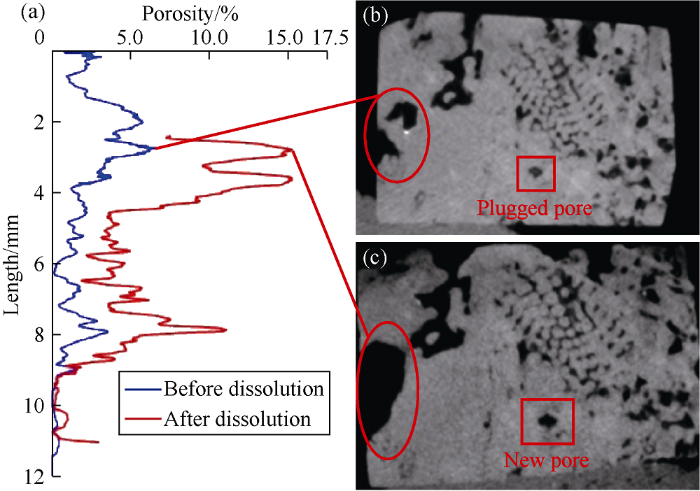
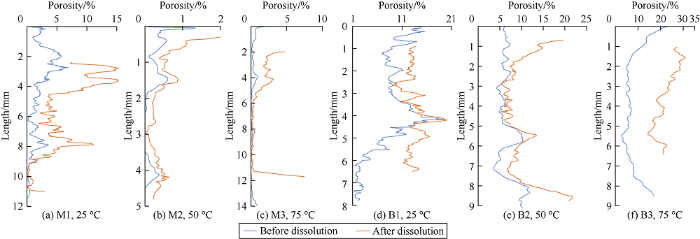
Porosity extracted from X-ray microCT analysis is defined as ratio of mapped pore volume and bulk volume of rock sample. It comprises isolated and connected pores. As expected, dissolution of carbonate rock enhances the overall porosity (Fig. 4). The porosity profiles before and after dissolution exhibit similar distribution characteristics in some sections along the samples, which is interpreted as enhancement of the initial pore due to dissolution (Fig. 4a). However, in some part of the porosity profile, porosity after dissolution greatly increases compared to the initial porosity profile. This important increase of porosity suggests a significant expansion of initial pore system. Dissolution of calcite results in dense pore wall where the diluted HCl solution can easily percolate because of the presence of unstable calcite minerals and large initial pore size. This dissolution can lead to very large cavities and new pore system (Fig. 4c), improving considerably the total porosity. Before dissolution, these newly formed pores were filled by fine and unstable calcite minerals. After dissolution, these materials have been dissolved and formed new pores.
Fig. 4.
Fig. 4.
Porosity distribution of carbonate sample before and after dissolution.
Fig. 5 shows the porosity changes of type 1 rock of samples M1, M2 and M3 and type 2 rock of samples B1, B2 and B3 at 25, 50, 75 °C respectively. Because of the heterogeneity of rock samples, different samples of type 1 rock and samples of type 2 rock are slightly different in initial porosity (before dissolution). During dissolution, porosity increases as expected. The porosity change is significant at the top of sample where there is continuous reacts with HCl solution by the stirrer, leading to faster dissolution than the other part of the rock. Porosity change occurs throughout the sample which implies that sample is dissolved in 3 directions along x-axis, y-axis and z-axis. However, the uniform porosity change throughout the sample indicates that the change is mainly dominated by pore enlargement rather than creation of new pore system.
Fig. 5.
Fig. 5.
Porosity variation induced by dissolution of two types of carbonate rocks at different temperatures.
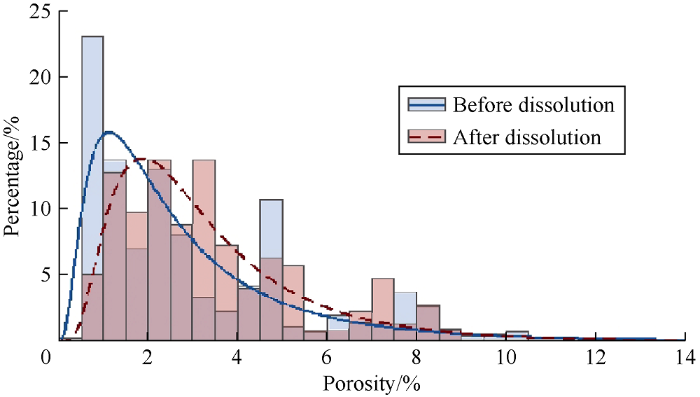
It can be seen from Fig. 6, porosity distribution is characterized by positive skewness. After dissolution at different temperatures, porosity distribution is still characterized by positive skewness but is slightly left skewed. After dissolution, kurtosis value decreases, implying that porosity distribution peak is flatter than before dissolution. Porosity range after dissolution also increases as compared to the porosity range before dissolution. In the type1 rock, porosity increases by 3.0, 4.0 and 4.5 times from the initial porosity at 25, 50, 75 °C respectively. However, these increment factors only correspond to 3.50%, 0.32% and 0.83% increase of porosity at 25, 50, and 75 °C respectively. In the type 2 rock, porosity only increases by 1.6, 1.6, and 2.8 times from the initial porosity, but these increment factors correspond to a significant increase of porosity of 4.7%, 4% and 14.3% at 25, 50, 75 °C respectively. At 25 °C and 50 °C, the porosity variations are similar whereas, at 75°C, the change is much more significant.
Fig. 6.
Fig. 6.
Histogram of porosity distribution before and after dissolution.
From these results, it can be inferred that the porosity increment factor increases as the temperature increases. Moreover, having a significant increment factor does not necessary mean high porosity variation. In this case, the initial porosity of carbonate rock contributes to the porosity enhancement by dissolution. Therefore, during dissolution, in porous carbonates such as grainstones, small increment factor can result in a significant porosity variation, whereas in less porous carbonate rocks such as mudstone, important increment factor can lead to small porosity variation. Therefore, the most significant porosity variation is obtained from the dissolution at the highest temperature of carbonate rock with high initial porosity.
2.2.2. Pore size distribution changes
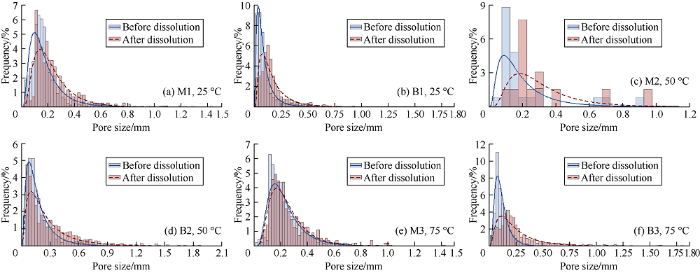
In the 2 types of carbonate rocks, the total numbers of pores vary from 25 (in the less porous mudstone) to 4139 (in the porous grainstone). After dissolution, these numbers decrease to 13-1637, which is in agreement with results provided in Reference [11]. The reduction in pore numbers suggest that pores are merged together forming larger pores. Fig. 7 shows the pore size distribution (PSD) of all samples before and after dissolution at 25, 50 and 75 °C. Pore size distributions of two types of carbonates are lognormal distribution with normal skewness. After dissolution, regardless of the temperature variations, pore size distributions still show positive skewness but are slightly skewed left, and the pore size distribution curve is flatter than the one before dissolution. The skewness of PSD after dissolution toward the higher value confirms that the increase of overall pore size. The decrease of kurtosis of PSD after dissolution suggests that the PSD is more spread around the median than the one before dissolution.
Fig. 7.
Fig. 7.
Pore size distribution variation of 2 types of carbonate rock at different temperatures.
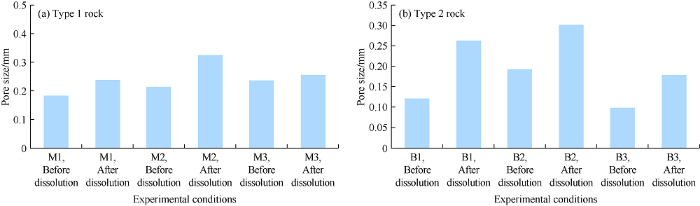
For type 1 rock, the dissolution at 25, 50 and 75 °C made pore size increase by 1.3, 1.5 and 1.1 times from the initial pore size, and made the pore diameter increase by 0.06, 0.11, 0.02 mm respectively (Fig. 8a). Dissolution has more significant effect on pore diameter of the type 2 rock than type 1 rock. At 25, 50 and 75 °C, pore diameter of type 2 rock increased by 1.80, 1.60 and 1.92 times, corresponding to 0.08, 0.11 and 0.11 mm respectively (Fig. 8b).
Fig. 8.
Fig. 8.
Pore size variation of 2 types of carbonate rock at different temperatures.
No correlation is observed between the temperature and pore size variation for the two rock types. On the other hand, the pore size variation of type 2 rock is larger than that of type 1, which indicates that the heterogeneity of the rock and existence of unstable carbonate minerals are the key factors affecting pore size enlargement.
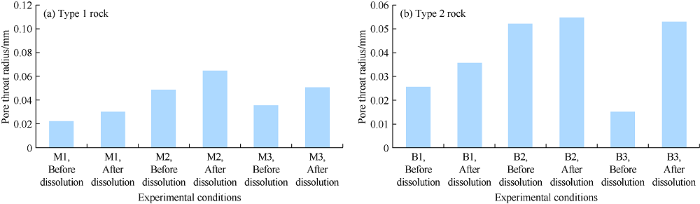
2.2.3. Variation of pore throat radius
Pore throat of carbonate rock is characterized by its radius and its length. Similar to pore size distribution, the best distribution fit for the pore throat radius distribution (PTRD) is lognormal distribution (Fig. 9). Both pore throat radius distribution before and after dissolution show positive skewness. However, after dissolution, the pore throat radius distribution is slightly left skewed, indicating an overall increase of pore throat size.
Fig. 9.
Fig. 9.
Pore throat radius distribution variation of 2 types of carbonate rock at different temperatures.
For type 1 rock, the average pore throat radius increased by 0.01 mm at 25 °C and 0.02 mm at 50 and 75 °C (Fig. 10a). The pore throat radius increased by 1.3 times at 50 °C, 1.4 times at 25 and 75 °C after dissolution. For type 2 rock, dissolution at 25, 50 and 75 °C make pore throat radius enlarge by 0.010, 0.003 and 0.040 mm respectively (Fig. 10b).
Fig. 10.
Fig. 10.
Pore throat radius variation of two types of 2 types of carbonate rock at different temperatures (using independent standard deviation to calculate the interval).
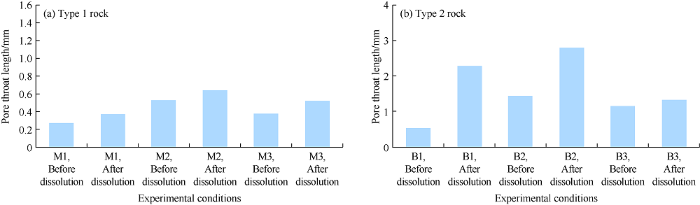
2.2.4. Variation in pore throat length
Similar to PTRD, the pore throat length also shows lognormal distribution, and both distributions are characterized by positive skewness before and after dissolution at different temperatures. The pore throat length distribution after dissolution skewed left than before dissolution, indicating the pore throat length increased. In addition, the peak of pore throat length after dissolution is slightly flatter and has more data in the tail than that before dissolution, and is characterized by a decrease of kurtosis value (Fig. 11).
Fig. 11.
Fig. 11.
Pore throat length distribution variation of 2 types of carbonate rock at different temperatures.
For the type 1 rock, the average pore length increased by 0.1 mm at 25 °C, 0.12 mm at 50 °C and 0.14 mm at 75 °C (Fig. 12a). For type 2 rock, this pore throat length increased by 0.10 mm at 25 °C, 0.05 mm at 50 °C and 0.35 mm at 75 °C (Fig. 12b). On the whole, pore throat length increased by 1.1 to 3.5 times after dissolution. These results suggest that temperature and heterogeneity of the samples are major factors controlling pore throat variation due to dissolution.
Fig. 12.
Fig. 12.
Variation of pore throat length of 2 types of carbonate rock at different temperatures.
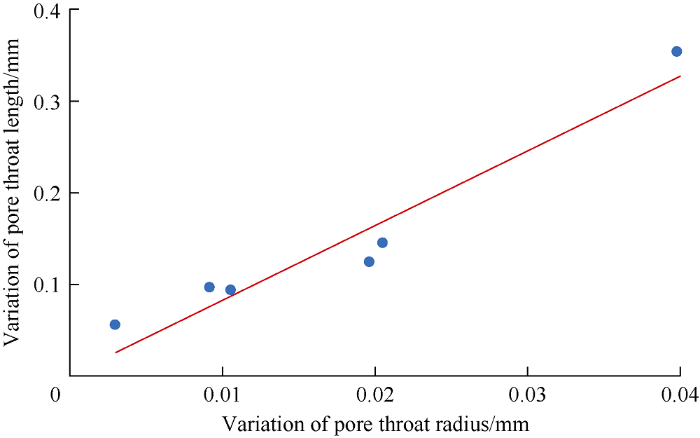
From analysis of pore throat attributes before and after dissolution, the variation of pore throat radius is proportional to the variation of pore throat length (Fig. 13), suggesting uniform development of the pore throat in 3 directions.
Fig. 13.
Fig. 13.
The relationship of variation of pore throat radius and variation of pore throat length.
2.2.5. Changes in coordination number
The coordination number is related to connectivity of pores. It defines the number of throats connected to each pore. Therefore, the higher the coordination number, the better the pore connectivity is. Before dissolution, for type 1 rock, the average coordination number of the 3 samples were 4.2 (M1), 1.0 (M2) and 1.2 (M3) at 25, 50 and 75 °C respectively. After dissolution, the coordination number changed to 5.3 (M1), 2.9 (M2) and 2.2 (M3) respectively. For type 2 rock, the coordination number of the 3 samples were 3.0 (B1), 2.7 (B2) and 4.2 (B3) at 25, 50 and 75 °C respectively, the coordination number after dissolution increased to 4.4 for B1 at 25 °C, 4.0 for B2 at 50 °C and 7.9 for B3 at 75 °C. The observation results show that the carbonate rock sample with highly connected initial pores would have the best connectivity after dissolution at the highest temperature.
2.3. Simulation of permeability before and after dissolution
In order to evaluate the effect of dissolution on fluid flow ability of the carbonate rocks, permeability before and after dissolution were simulated. For type 2 rock, the simulated permeability changed significantly. For B1 after dissolution at 25 °C, the simulated permeability increased by 1.3 times, from 751.5×10-3 μm2 to 950.0×10-3 μm2 (increase of 198.5× 10-3 μm2). For B2 after dissolution at 50 °C, the permeability increased from 843.3×10-3 μm2 to 2358.3×10-3 μm2 (increase of 1514.9×10-3 μm2) by 2.8 times. For B3 after dissolution at 75 °C, the permeability increased by 3.4 times than the initial permeability, from 1639×10-3 μm2 to 5566.5×10-3 μm2 (increase of 3928×10-3 μm2). For type 1 rock, After dissolution at 25, 50 and 75 °C, the permeability increased from 13.04× 10-3, 4.80×10-3, 2.50×10-3 μm2 to 50.00×10-3, 17.75×10-3, 9.45×10-3 (that is an increment of 36.96×10-3, 12.95×10-3, 6.95×10-3 μm2) respectively. Simulated permeability of the two types of carbonate rocks both increased significantly after dissolution. But the variation of permeability induced by dissolution is more significant in type 2 rock with higher initial permeability than type 1, suggesting that the permeability increase caused by dissolution is relatively selective. Therefore, dissolution is more effective in permeable carbonate rock than in less permeable carbonate rock due to connectivity of pore networks. Moreover, the analysis of pore throat attributes changes, coordination number and the variation of simulated permeability (Table 2) suggests that small changes of pore attributes like the variation of 0.01 mm in pore radius, 0.1 mm in pore length and slight variation in coordination number (one unit) can lead to very significant permeability changes of an order of 1 000×10-3 μm2 in porous carbonate reservoir rock and 10×10-3 μm2 in less porous reservoir.
3. Conclusions
In this study, the change of released Ca2+ concentration was described by the dissolution kinetics model of carbonate rock. The average dissolution rate is directly related to temperature for all samples tested. The study shows that the dissolution process that modifies pore systems differently and complicatedly for each sample, and the more porous carbonate rock dissolved at the high temperature has the most significant porosity variation. The comparison between pore size distribution before and after dissolution at different temperatures indicates that there is no correlation between the temperature and pore size variation. However, pore size variation in type 2 rock is larger than the variation in the Type 1, indicating that the pore size variation is mainly controlled by the physical property (initial porosity and permeability) of the rock itself and the abundance of unstable minerals (function of crystal shape, size and mineral type) at the pore wall. Pore throat attributes (radius and length) are also affected by dissolution. However, the pore throat radius variation at different temperatures are very small, ranging from 0.003 mm to 0.040 mm and with an average increment factor of 1.7 for the studied rock types. Pore throat length also had small variations, ranging from 0.05 to 0.35 mm. The pore throat attributes generally increase as the temperature of dissolution increases. After dissolution, coordination number representing the connectivity of pore networks increased. The carbonate rocks with higher initial coordination number would have better pore connectivity after dissolution at high temperature. Permeability increases as the temperature increases from the analysis of permeability simulation before and after dissolution at different temperatures. Variation of permeability is more significant in porous carbonate rocks than in less porous carbonate rock. Furthermore, it is found that minor changes of the pore throat radius, length and connectivity have great impact on permeability. The results of this study give us a better understanding on carbonate reservoir behavior at pore-scale caused by fluid-solid interactions. This information can be useful when implementing EOR such as acidizing at specific zones of the reservoir, although the experiment condition is static acidizing.
Nomenclature
Cca—Ca2+ concentration, 10-6;
Cca,0—Ca2+ concentration at t=1 min, 10-6;
DR*mean—average dissolution velocity, 10-6/min;
k—rate constant, 10-6/min;
r—instantaneous dissolution velocity, 10-6/min;
t—time, range from 0 to100, min.
Reference
Experimental determination of porosity and permeability changes induced by injection of CO2 into carbonate rocks
DOI:10.1016/j.chemgeo.2009.03.028 URL [Cited within: 2]
Kinetics of carbonate dissolution and its effects on the porosity and permeability of consolidated porous media
DOI:10.1016/j.petrol.2013.11.015 URL [Cited within: 1]
Fracture acidizing in carbonate rock
Understanding wormholes in carbonates: Unprecedented experimental scale and 3D visualization
Simulation and analysis of wormhole propagation by VES acid in carbonate acidizing
DOI:10.1016/j.petrol.2015.12.011 URL [Cited within: 1]
Accounting for sequestered carbon: The question of permanence
An issue of permanence: Assessing the effectiveness of temporary carbon storage
DOI:10.1023/A:1024801618900 URL [Cited within: 1]
Dynamic three-dimensional pore-scale imaging of reaction in a carbonate at reservoir conditions
DOI:10.1021/es505789f URL [Cited within: 2]
Calcium carbonate dissolution rate in limestone contactors
An experimental study of calcite and limestone dissolution rates as a function of pH from -1 to 3 and temperature from 25 °C to 80 °C
DOI:10.1016/S0009-2541(98)00080-1 URL [Cited within: 1]
Modeling of the effect of pH on the calcite dissolution kinetics. Theoretical Foundations of
Effect of intensity and pH of rain on the dissolution of limestone: The Nineteenth International Offshore and Polar Engineering Conference
Dissolution kinetics of Devonian carbonates at circum-neutral pH, 50 bar pCO2, 105 °C, and 0.4 M: The importance of complex brine chemistry on reaction rates
DOI:10.1016/j.apgeochem.2013.12.008 URL [Cited within: 1]
Experimental determination of natural carbonate rock dissolution rates with a focus on temperature dependency
DOI:10.1016/j.geomorph.2016.02.019 URL [Cited within: 1]
From pore scale to wellbore scale: Impact of geometry on wormhole growth in carbonate acidization
DOI:10.1016/j.ces.2008.03.021 URL [Cited within: 1]
Effect of medium heterogeneities on reactive dissolution of carbonates
DOI:10.1016/j.ces.2008.10.026 URL [Cited within: 1]
Characterization of pore systems in carbonate using 3D X-ray computed tomography
Real-time 3D imaging of Haines jumps in porous media flow
DOI:10.1073/pnas.1221373110 URL [Cited within: 1]
X-ray imaging and analysis techniques for quantifying pore-scale structure and processes in subsurface porous medium systems
DOI:10.1016/j.advwatres.2012.07.018 URL [Cited within: 1]
Pore-scale imaging and modelling
DOI:10.1016/j.advwatres.2012.03.003 URL [Cited within: 1]
Electrical and flow properties of highly heterogeneous carbonate rocks
DOI:10.1306/05221312134 URL [Cited within: 1]
Numerical modeling of fluid and electrical currents through geometries based on synchrotron X-ray tomographic images of reservoir rocks using Avizo and COMSOL
Multi-scale, micro-computed tomography-based pore network models to simulate drainage in heterogeneous rocks
DOI:10.1016/j.advwatres.2015.02.003 URL [Cited within: 1]
2D and 3D imaging resolution trade-offs in quantifying pore throats for prediction of permeability
DOI:10.1016/j.advwatres.2013.08.010 URL [Cited within: 1]
Estimation of 3-D pore network coordination number of rocks from watershed segmentation of a single 2D image
DOI:10.1016/j.advwatres.2016.05.020 URL [Cited within: 1]
Pore network modeling: Analysis of pore size distribution of Arabian core samples
Pore structure characterization of Indiana limestone and pink dolomite from pore network reconstructions
3D analysis of geometry and flow changes in a limestone fracture during dissolution
DOI:10.1016/j.jhydrol.2013.01.035 URL [Cited within: 1]
Changes in porosity, permeability, water retention curve and reactive surface area during carbonate rock dissolution
DOI:10.1016/j.chemgeo.2015.03.008 URL [Cited within: 1]
Influence of transport and reaction on wormhole formation in porous media
DOI:10.1002/(ISSN)1547-5905 URL [Cited within: 1]
Alternative stimulation fluids and their impact on carbonate acidizing
Optimum conditions for wormhole formation in carbonate porous media: Influence of transport and reaction
Acidizing carbonate reservoirs: Numerical modelling of wormhole propagarion and comparison to experments
Pore network modelling of reactive transport and dissolution in porous media
DOI:10.1007/s11242-016-0695-x URL [Cited within: 1]
Methods of obtaining and analyzing kinetic data
Kinetics and rate-limiting mechanisms of dolomite dissolution at various CO2 partial pressures.
DOI:10.1007/BF02880680 URL [Cited within: 1]