Introduction
Saturated saltwater drilling fluid (SSDF) is based on bentonite, salt-tolerant polymer and inorganic salt[1,2,3], which possesses excellent tolerance of salt contamination and anti- sloughing performance, and is suitable for strata with high salinity and water sensitivity. With the irreplaceable superiority, it is not only the first choice for drilling in gypsum salt beds[4], but also the future development direction of high-performance water-based drilling fluid for shale gas reservoir[5]. Essentially, the performance of SSDF is dependent upon the performance of the salt-tolerant polymer in it. On the one hand, the polymer functions uppermost to adjust rheological properties and fluid loss and wall-building; on the other hand, although SSDF’s performance can be improved by increasing polymer addition, using sulfonated materials and diverse compatible additives, these methods would bring about high cost, environmental issues and difficult maintenance etc[2, 4]. As SSDF develops towards high-temperature resistance and high density, study on salt-tolerant polymer becomes increasingly important[6,7].
The reason that conventional polymer additives work badly in saline environment is the polyelectrolyte effect[8]. Under high salinity, the screening effect of small ions weakens electrostatic forces between charged groups, which makes conformation of polymer chain turn from extension to shrinkage, thus the polymer cannot keep hydration dispersion, and become poorer in performance and even insoluble. Weakening polyelectrolyte effect is an effective way to improve salinity tolerance of polymer. The most commonly-used method is to introduce sulfonated monomers, like 2-acrylamido-2-methylpropane sulfonate acid and styrene sulfonic acid[9,10] into polymer, which can make the polymer maintain moderate hydration under salinity because of strong hydrophilicity of sulfonic groups[11,12]. Comprehensively, though these polymers are salt-tolerant, they are not compatible with salt in essence. Dispersions of these polymers with salt are thermodynamically unstable, thus they cannot long resist high-salinity and high-temperature. Studies have indicated that polyampholyte (polyzwitterion) with equivalent anionic and cationic charge is a kind of responsive polymer with excellent salt-tolerance. In contrary to normal polymer, it displays positive responsiveness to salt owing to antipolyelectrolyte effect, in other words, its conformation turns from shrinkage to extension under salt stimulus and its performance improves with the rise of salt content[13,14]. Obviously, the change from salt- resistance to salt-amity makes polymer synergetic with instead of resistant to salt. In other words, the polymer is naturally compatible with salt, thus it can maintain long-time stability under high-salinity.
Salt-responsive polyampholyte is usually synthesized using inner-salt monomer[13], but it is too simple in configuration and high in cost. In drilling fluid, considering rheology modification, fluid loss control and interaction with bentonite, polymer additive should have suitable flexibility, molecular weight, ion density and charge distribution[15,16]. In addition, considering the convenience when using in the field, especially the problem of dissolving high-molecular-weight polymer, solid polymer powder should be prepared into liquid. In this study, we prepared two kinds of salt-responsive polyampholytes by inverse emulsion polymerization. Their responsiveness to salt was studied by analyzing the rheology of their solution, and they were compared with two kinds of salt-tolerant polymers commonly used in drilling fluid. At last, a high-performance SSDF based on the salt-responsive polyampholytes was prepared and evaluated.
1. Preparation of salt-responsive polyampholytes
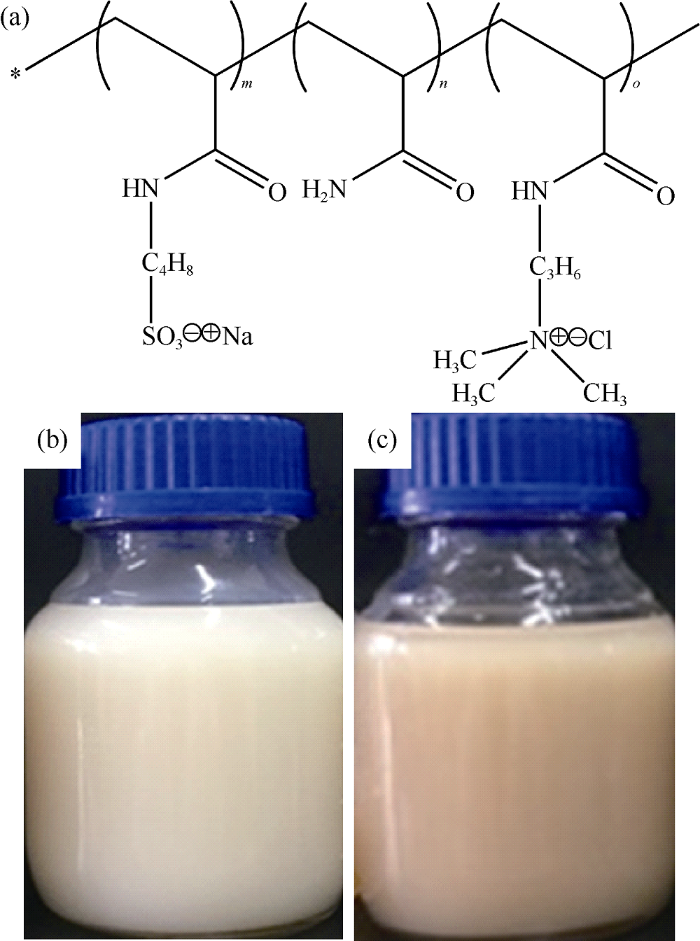
The salt-responsive polyampholytes were synthesized by copolymerization of nonionic monomer acrylamide (AM), anionic monomer 2-acrylamido-2-methylpropane sulfonate acid (AMPS) and cationic monomer 3-acrylamidopropyl trimethylammonium chloride (TAC) of chemically-pure. By changing molar feed ratio of the three monomers, one polymer with high molecular weight and low charge density (HvL) and the other with low molecular weight and high charge density (LvH) were prepared by inverse emulsion polymerization, respectively (their molecular structures are shown in Fig. 1a). The feed ratio of the three monomers of them are 9.0:0.5:0.5 and 4:3:3 respectively.
Fig. 1.
Fig. 1.
Molecular structures of salt-responsive polyampholytes (a) and appearance of HvL (b) and LvH (c).
The procedure of inverse emulsion polymerization is as follows. 90 g of the three monomers were dissolved in 120 g of deionzed water, and the pH of the solution was adjusted to 7-8 using NaOH, then 0.9 g of Tween-80 was added and dissolved to get the water phase. 12.5 g of Span-80 was dissolved in 75 g of 5# mineral oil to get the oil phase. The two phases were mixed and blended using a homogenizer at high speed for 5 minutes to obtain the inverse emulsion. The emulsion was poured into a three-neck flask and 0.18 g of initiator, 2,2'-azobis (2,4-dimethyl) valeronitrile (analytically-pure) was added. A water bath and a power-driven blender were equipped. Nitrogen was bubbled through the emulsion for 15 min and then the flask was sealed and bath temperature was elevated to 50 °C. The product was obtained after 20 h of reaction at moderate blending speed. The appearances of HvL and LvH are shown in Fig. 1b and 1c.
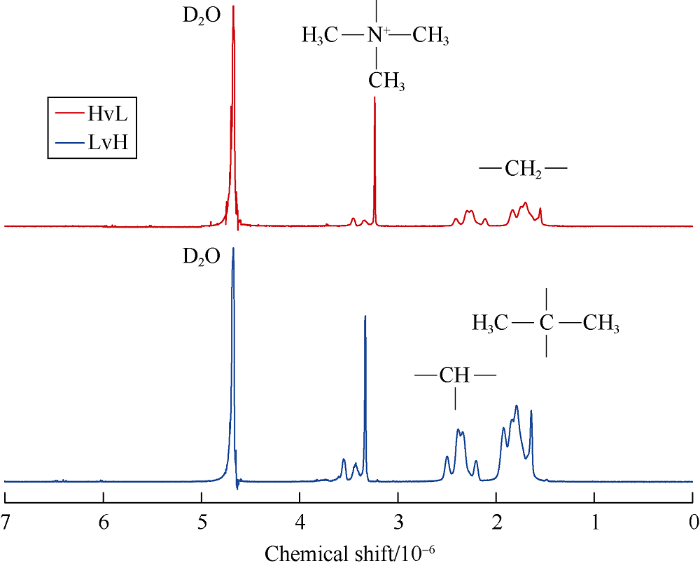
After demulsification, washing and drying process, the structure of the polymers were characterized by proton nuclear magnetic resonance (1H-NMR) using deuterated water (D2O). It can be seen from Fig. 2 that spectrum of HvL and that of LvH are basically identical since the polymers are synthesized using the same monomers and method. For example, in spectrum of HvL, peak at 1.58×10-6 represents hydrogen atom (H) on methyl group of AMPS, peaks at (1.70-1.84)×10-6 and (2.11-2.41)×10-6 represent H on methylene group and methine group in the polymer’s backbone, pointed peak at 3.23×10-6 represents H on methyl group connected to N+ of TAC. These characteristic peaks prove the successful polymerization of the three monomers. Meanwhile, no peaks of H on propenyl group occur, indicating the low content of monomer residue and thus high conversion.
Fig. 2.
Fig. 2.
1H-NMR spectra of the salt-responsive polyampholytes.
2. Rheology of salt-responsive polyampholyte solution
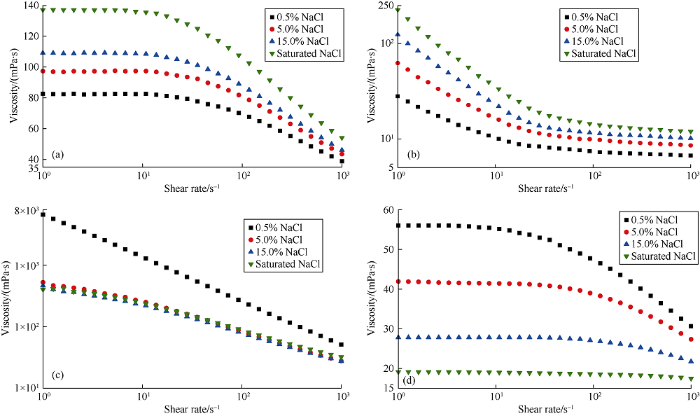
The most obvious feature of salt-responsive polyamo-pholyte is that its chain conformation extends as salt content increases. The chain extension increases excluded volume, leading to viscosity increase of the polymer solution. In order to characterize salt-responsiveness, solutions of HvL and LvH at concentration of 2% (in mass fraction) were prepared, and viscosity variation of the solutions with the increase of NaCl content were measured using a HAKKE rheometer (MARS60, Thermo Fisher Scientific USA). From Fig. 3a, it can be seen that viscosity of HvL solution increases with the increase of NaCl content, and its shear thinning is improved in high- shear-rate area, but shows a newtonian plateau in low-shear- rate area. The viscosity increase is also observed in LvH solution (Fig. 3b), differently, its newtonian plateau emerges in high-shear-rate area. Such result not only directly proves salt-responsiveness of HvL and LvH, but also reveals influences of molecular weight and charge density on rheology. Owing to high molecular weight, HvL molecule is hard to move, and thus insensitive to low amplitude shear, so its viscosity maintains constant basically under low shear rate. Lower in molecular weight, LvH can be dispersed fully at slightly higher shear rate, thus, its viscosity maintains basically constant under high shear rate. But its viscosity increases under low shear rate because of its microstructure formed by aggregation driven by electrostatic forces.
Fig. 3.
Fig. 3.
Influences of NaCl content on viscosity of HvL (a), LvH (b), AM-AMPS anionic copolymer (c) and AM-AMPS-DMDAAC polyampholyte solutions.
Two commonly-used salt-tolerant polymers were compared with HvL and LvH. At the same feed ratio of HvL, AM- AMPS anionic copolymer was synthesized by removing cationic monomer TAC and AM-AMPS-DMDAAC polyampholyte was synthesized by replacing TAC for equivalent loading of cationic monomer diallydimethyl ammonium chloride (DMDAAC). Because AMPS and DMDAAC are commonly used monomers to prepare salt-tolerant and heat-tolerant polymer additives, and anionic and amphoteric are two main copolymer types used in SSDF, such comparison is representative. It can be seen from Fig. 3c and 3d that AM- AMPS anionic copolymer displays typical polyelectrolyte effect, in other words, viscosity of its solution decreases with the rise of NaCl content. At the NaCl adding amount of 5%, viscosity of its solution basically drops to the lowest level. AM-AMPS-DMDAAC polyampholyte does not show antipolyelectrolyte effect either, viscosity of its solution decreases with the rise of NaCl content. These results not only show the specialty of HvL and LvH, but also indicate the influence of polymer configuration on salt-responsiveness. For AM-AMPS- DMDAAC polyampholyte, the equivalent feed of anionic and cationic monomer cannot yield neutral structure of polyzwitterion, because of the big difference between monomer structure (DMDAAC forms heterocyclic structure after polymerization) and the regular polymerization process[14].
3. Performance evaluation of salt-responsive polyampholytes
Performance of the salt-responsive polyampholytes in a base mud with 2% bentonite and saturated NaCl was evaluated. Table 1 shows that the base mud flocculated severely before HvL or LvH was added, as plenty of salt screened electrostatic interaction between bentonite particles, resulting in high Φ6/Φ3 readings and uncontrollable fluid loss. Flocculation also causes bentonite settlement, base mud stratification and instability. Both HvL and LvH kept bentonite in good dispersion in high-salinity environment, decreasing API fluid loss to 4.1 mL and 22.0 mL, respectively. In terms of rheology modification, the HvL with higher molecular weight can increase viscosity and yield point of the mud, while high charge density LvH is able to maintain moderate Φ6/Φ3 readings for base mud at low viscosity.
Table 1 Influence of HvL and LvH on basic performance of base bentonite mud with saturated salt.
| Sample | Apparent viscosity/ (mPa·s) | Plastic viscosity/ (mPa·s) | Yield point/ Pa | Φ6/Φ3 | API fluid loss/mL |
|---|---|---|---|---|---|
| Base mud | 8.5 | 6 | 2.56 | 8/7 | 139.8 |
| Base mud + 2% HvL | 52.0 | 31 | 21.46 | 5/4 | 4.1 |
| Base mud+ 2% LvH | 18.0 | 12 | 6.13 | 4/3 | 22.0 |
Note: Φ6 and Φ3 are readings of rotary viscometer at rotation speed of 6 r/min and 3 r/min, respectively.
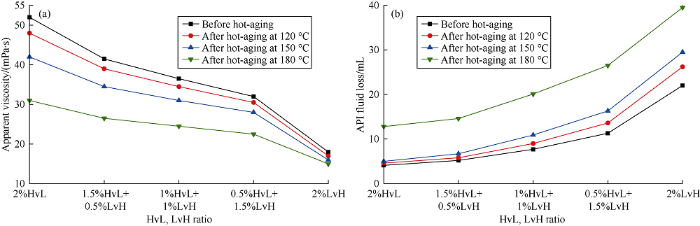
Generally, the viscosity of polymer drilling fluid is inversely proportional to its fluid loss. As both high viscosity and high fluid loss are not desired, the ratio between HvL and LvH should be adjusted to balance viscosity and fluid loss. The effects of their ratio on apparent viscosity and API fluid loss of base mud with polymer content of 2% under different hot-aging temperatures were tested, to optimize their adding amount in SSDF and to evaluate heat-tolerance of the mud (Fig. 4). It can be seen that with the decrease of HvL’s ratio, apparent viscosity decreases whereas fluid loss increases gradually. Data obtained after hot-aging test indicates that both HvL and LvH can resist temperature of 150 °C under saturated salinity. Combined with data in Table 1, we can see that both HvL and LvH work well in adjusting rheology and controlling fluid loss. In comparison, HvL is more of a fluid loss additive with thickening effect while LvH is a low-viscosity fluid loss control additive. Considering viscosity adjustment and fluid loss control, the HvL with lower cost (high AM content) should be used preferentially in drilling fluid.
Fig. 4.
Fig. 4.
Effects of ratio of HvL and LvH on apparent viscosity (a) and API fluid loss (b) of base saturated salt bentonite mud.
4. SSDF based on salt-responsive polyampholytes
4.1. Formula and basic performance
Based on excellent compatibility between HvL and LvH with salt and their functions of modifying rheology and decreasing fluid loss, a SSDF centering on them was prepared with superfine CaCO3. The formula was 2% bentonite+1% HvL+0.5% LvH+4% CaCO3 (particle size of 6.5 μm; 2000 mesh) + saturated NaCl+150% barite (density of 2.0 g/cm3). CaCO3 is an inactive plugging agent and able to improve filter cake quality. It can be seen from Table 2 that the SSDF based on salt-responsive polyampholytes has favorable viscosity, gel strength, high dynamic plastic ratio and low fluid loss. Its rheological property basically maintains constant before and after hot-aging, indicating excellent basic performance.
Table 2 Basic performance of SSDF based on HvL and LvH.
| Condition | Apparent viscosity/(mPa·s) | Plastic viscosity/ (mPa·s) | Yield point/Pa | Dynamic plastic ratio/ (Pa·(mPa·s)-1) | Φ6/Φ3 | API fluid loss/mL | Fluid loss under high temperature and pressure/mL |
|---|---|---|---|---|---|---|---|
| Before hot-aging After hot-aging | 80 79 | 66 66 | 14.31 13.29 | 0.217 0.201 | 6/4 5/4 | 2.1 | |
| 8.0 |
Note: All the hot-aging experiments were done at 150 °C for 16 h, the same below. All the fluid loss under high temperature and pressure tests were done at 150 °C and 3.5 MPa, the same below.
Based on this formula, with identical polymer dosage, same kinds and addition of other additives, SSDFs with AM-AMPS anionic copolymer and AM-AMPS-DMDAAC polyampholyte were prepared to compare. From Tables 3 and 4, the comparison drilling fluids reduce significantly in viscosity after 16 h of hot-aging, with fluid loss goes out of control at high temperature and high pressure. Apparently, both the drilling fluids with the comparison polymers are not suitable for the conditions with saturated salt and high-temperature (150 °C), and perform far worse than the drilling fluid with salt-responsive polyampholytes.
Table 3 Basic performance of SSDF prepared with AM-AMPS anionic copolymer.
| Condition | Apparent viscosity/(mPa·s) | Plastic viscosity/ (mPa·s) | Yield point/Pa | Dynamic plastic ratio/ (Pa·(mPa·s)-1) | Φ6/Φ3 | API fluid loss/mL | Fluid loss under high temperature and pressure/mL |
|---|---|---|---|---|---|---|---|
| Before hot-aging After hot-aging | 86.5 49.0 | 70 47 | 16.86 2.04 | 0.241 0.043 | 7/5 2/1 | 3.9 | |
| 53.3 |
Table 4 Basic performance of SSDF prepared with AM-AMPS-DMDAAC polyampholyte.
| Condition | Apparent viscosity/(mPa·s) | Plastic viscosity/ (mPa·s) | Yield point/Pa | Dynamic plastic ratio/ (Pa·(mPa·s)-1) | Φ6/Φ3 | API fluid loss/mL | Fluid loss under high temperature and pressure/mL |
|---|---|---|---|---|---|---|---|
| Before hot-aging After hot-aging | 64.0 38.5 | 58 36 | 6.13 2.56 | 0.106 0.071 | 5/4 4/3 | 5.1 | |
| 68.8 |
4.2. Thermal stability
Continuous hot-aging experiment at 150 °C was conducted to evaluate thermal stability of SSDF based on salt-responsive polyampholytes. It can be seen from Table 5 that with the growth of hot-aging time, viscosity and Φ6/Φ3 readings decrease while fluid loss increases gradually. Combined with data in Table 2, the drilling fluid performance become worse after aging for 80 h, which indicates excellent thermal stability of SSDF.
Table 5 Variation of basic properties of SSDF based on HvL and LvH with hot-aging time.
| Hot-aging time/h | Apparent viscosity/ (mPa·s) | Plastic viscosity/(mPa·s) | Yield point/Pa | Dynamic plastic ratio/(Pa·(mPa·s)-1) | Φ6/Φ3 | Fluid loss under high temperature and pressure/mL |
|---|---|---|---|---|---|---|
| 32 64 80 96 | 75.5 68.0 62.0 54.0 | 64 57 53 47 | 11.75 11.24 9.20 7.15 | 0.184 0.197 0.174 0.152 | 5/4 4/3 3/2 3/2 | 10.0 11.8 15.4 23.0 |
4.3. Tolerance to bentonite and shale cutting contamination
Tables 6 and 7 present basic properties of SSDF after contaminated by different contents of bentonite and shale cutting (after hot-aging at 150 °C for 16 h). It can be seen that the drilling fluid has good inhibition effect because of its high content of salt. From variation of Φ6/Φ3 readings, it can be seen that considerable thickening occurs only when it is contaminated by over 30% of bentonite in drilling fluid. Shale cutting has little influence on the drilling fluid’s performance. At 30% of shale cutting contamination, it only slightly increases in viscosity and fluid loss.
Table 6 Tolerance of SSDF based on HvL and LvH to bentonite contamination (after hot-aging).
| Contamination quantity/% | Apparent viscosity/(mPa·s) | Plastic viscosity/(mPa·s) | Yield point/Pa | Dynamic plastic ratio/(Pa·(mPa·s)-1) | Φ6/Φ3 | Fluid loss under high temperature and pressure/mL |
|---|---|---|---|---|---|---|
| 0 5 15 25 30 | 79 83 88 117 131 | 66 71 75 96 94 | 13.29 12.26 13.29 21.46 37.81 | 0.201 0.172 0.177 0.224 0.402 | 5/4 5/4 6/5 9/7 21/18 | 8.0 10.0 11.8 15.4 23.0 |
Table 7 Tolerance of SSDF based on HvL and LvH to shale cutting (after hot-aging).
| Contamination quantity/% | Apparent viscosity/(mPa·s) | Plastic viscosity/(mPa·s) | Yield point/Pa | Dynamic plastic ratio/(Pa·(mPa·s)-1) | Φ6/Φ3 | Fluid loss under high temperature and pressure/mL |
|---|---|---|---|---|---|---|
| 0 10 20 30 | 79 82 89 100 | 66 69 75 84 | 13.29 13.29 14.31 16.35 | 0.201 0.192 0.191 0.195 | 5/4 5/4 6/5 9/7 | 8.0 8.2 10.3 13.2 |
4.4. Tolerance to CaCl2 contamination
Table 8 shows basic properties of SSDF after contaminated by CaCl2 of different contents (hot-aging at 150 °C for 16 h). From the viscosity and fluid loss under high temperature and pressure data, it can be seen that drilling fluid is tolerant to 0.75% of CaCl2 contamination. Further increase of CaCl2 contamination causes significant increase of fluid loss under high temperature and pressure[16], because AM produces carboxyl groups after thermal degradation which are coagulated by massive Ca2+.
Table 8 Tolerance of SSDF based on HvL and LvH to CaCl2 (after hot-aging).
| Contamination quantity/% | Apparent viscosity/(mPa·s) | Plastic viscosity/(mPa·s) | Yield point/Pa | Dynamic plastic ratio/(Pa·(mPa·s)-1) | Φ6/Φ3 | Fluid loss under high temperature and pressure/mL |
|---|---|---|---|---|---|---|
| 0 0.50 0.75 1.00 | 79.0 74.0 65.5 38.0 | 66 65 58 32 | 13.29 9.20 7.66 6.13 | 0.201 0.142 0.132 0.192 | 5/4 5/4 4/3 3/2 | 8.0 12.3 18.9 76.7 |
4.5. Saturated KCl drilling fluid
KCl drilling fluid is more widely-used in harsh strata because of the special inhibition effect of K+[17]. Saturated KCl drilling fluid was prepared with KCl instead of NaCl, with identical category and addition of other additives. Its performance is shown in Table 9. Comparing with data in Tables 2 and 9, it can be seen that due to stronger inhibition effect of K+, the drilling fluid saturated with KCl has lower viscosity and slightly higher fluid loss than drilling fluid saturated with NaCl. But it still has excellent and stable performance. This indicates that HvL and LvH have no distinct selectivity to regular monovalent cations, owning universality.
Table 9 Basic properties of drilling fluid based on HvL and LvH saturated with KCl.
| Condition | Apparent viscosity/(mPa·s) | Plastic viscosity/ (mPa·s) | Yield point/Pa | Dynamic plastic ratio/(Pa·(mPa·s)-1) | Φ6/Φ3 | API fluid loss/mL | Fluid loss under high temperature and pressure/mL |
|---|---|---|---|---|---|---|---|
| Before hot-aging After hot-aging | 69.0 66.5 | 58 56 | 12.24 10.73 | 0.194 0.192 | 5/3 5/3 | 3.0 | |
| 9.7 |
5. Conclusions
Salt-responsive polyampholytes, HvL and LvH, were synthesized with nonionic acrylamide, anionic 2-acrylamido-2-methylpropanesulfonate and cationic 3-acrylamidopropyl trimethylammonium chloride. HvL and LvH display obvious antipolyelectrolyte effect, which are compatible to salt naturally. In environment of saturated salt, both of them have effective functions of modifying rheology and decreasing fluid loss.
HvL and LvH are emulsion additives and can be easily prepared. With simple formula, good thermal stability, excellent inhibition effect and certain tolerance to calcium, the saturated saltwater drilling fluid centering on salt-responsive polyampholytes possesses high practical value for field applications.
Reference
Study on a new high temperature high density saltwater drilling fluid
Application of high temperature and high density saturated salt water drilling fluid in West Sichuan
A novel drilling fluid with less free water
Maintenance technology of high-density saturated brine drilling fluid during drilling into salt-gypsum layer
Status quo of water base drilling fluid technology for shale gas drilling in China and abroad and its developing trend in China
A solids-free brine drilling fluid system for coiled tubing drilling
DOI:10.1016/S1876-3804(18)30056-9 URL [Cited within: 1]
Synthesis and property evaluation of a salt- and alkali-resistant star-polymer
DOI:10.1016/S1876-3804(10)60049-3 URL [Cited within: 1]
Associating copolymer AM/DMDAAC/BA/AMPS as a tackifier in clay-free and water- based drilling fluids
Order-disorder conformational transitions of N-isopropylacrylamide-sodium styrene sulfonate copolymers in aqueous solutions
DOI:10.1021/ma800900v URL [Cited within: 1]
Iron oxide nanoparticles grafted with sulfonated copolymers are stable in concentrated brine at elevated temperatures and weakly adsorb on silica
Solubility of highly charged anionic polyelectrolytes in presence of multivalent cations: Specific interaction effect
DOI:10.1007/s101890050009 URL [Cited within: 1]
Responsive stabilization of nanoparticles for extreme salinity and high-temperature reservoir applications
Salt-responsive bilayer hydrogels with pseudo double network structure actuated by polyelectrolyte and anti-polyelectrolyte effects
Application of ionic liquid and polymeric ionic liquid as shale hydration inhibitors
An amphoteric polymer as anti-calcium contamination fluid loss additive in water-based drilling fluids
Application of organic salt/polyglycol/KCl drilling fluid