Introduction
Tight and low permeability reservoirs with huge recoverable resources have become a focus of petroleum exploration and development and the key replacement resource for the sustainable development of oil and gas industry in China[1,2]. For water-wet tight reservoirs, the injected water after hydraulic fracturing can not only displace the oil in fractures, but also the oil in tight matrix by imbibition[3,4]. But under the influences of acidic substances in crude oil, most tight reservoirs are oil-wet, and the water imbibition is inhibited by the capillary force, resulting in low imbibition recovery[5,6]. In recent years, to effectively produce the oil phase in tight matrix, many ways to enhance oil recovery (EOR) in oil-wet tight reservoirs have been studied, mainly including surfactant solution huff-and-puff, carbon dioxide huff-and-puff, and nanofluid displacement, etc.[7,8,9,10] Widely applicable and mature, surfactant huff and puff has been applied in Yates, Mauddud, Cottonwood Creek and other tight oil reservoirs in the US[11].
Initially, surfactant systems able to form in-situ microemulsion with crude oil were used as oil displacing agents in conventional oil reservoirs[12]. With continuous research, various commercial surfactant systems with low surfactant concentration (less than 0.2%) and low cosolvent concentration (less than 0.5%) have been successfully synthesized, suitable for reservoirs with harsh conditions and crude oil of different properties[13]. Results from core imbibition experiments and field practice both validated that this kind of surfactant system can effectively improve oil recovery in oil-wet tight reservoirs[14]. Data from Li’s experiments[15] also shows that the imbibition recovery was higher than 70% in small tight cores (length of 10 cm, diameter of 3.78 cm) and 38% in large tight cores (length of 30 cm, diameter of 9.8 cm), proving the compounded system can obtain higher recovery than mere surfactant. Furthermore, Li et al.[14] also compared the imbibition efficiency of different types of (Winsor Ⅰ and Winsor Ⅱ) surfactant systems, the results show that the oil recovery of Winsor Ⅰ type surfactant system (or refered as WI system, surfactant of this system could form Winsor Ⅰ type microemulsion with cosolevent solution) is higher than Winsor Ⅱ type surfactant system, implying the Winsor Ⅰ type surfactant has better application prospects in tight oil reservoir development.
To date, the optimization of surfactant, field adaptability evaluation cross-scale imbibition equation have been widely studied[16,17,18], while the dynamic phase behavior of in-situ microemulsion under flow conditions and imbibition mechanisms of microemulsion haven’t been examined in-depth. Besides, as an important variable during the optimization of surfactant system, the influence of salinity on imbibition efficiency has not been well understood.
In view of the above issues, NaCl concentration scan experiment, wettability identification and microfluidic experiment were conducted to study the phase change behavior of Winsor Ⅰ type and imbibition mechanisms.
1. Experiments
The experiment includes three parts, NaCl concentration scan experiment, wettability identification and microfluidic experiment.
1.1. Equipment
Leica M165FC microscope, Leica CCD camera (100 fps, 2 560×1 920 Pixel), neMESYS injection pump, and analytical balance etc.
1.2. Materials
Kerosene was used as oil phase, and DI water was used as water phase, sodium dodecyl sulfate as surfactant and N-butanol as cosolvent.
1.3. Experimental design
1.3.1. Surfactant system
Sodium dodecyl sulfate and N-butanol was mixed to form WI system. The ratio of surfactant to cosolvent was 1:1.5 for less balance time and more soluble oil, and the concentration of surfactant was 86.5 g/L.
1.3.2. Wettability characterization model
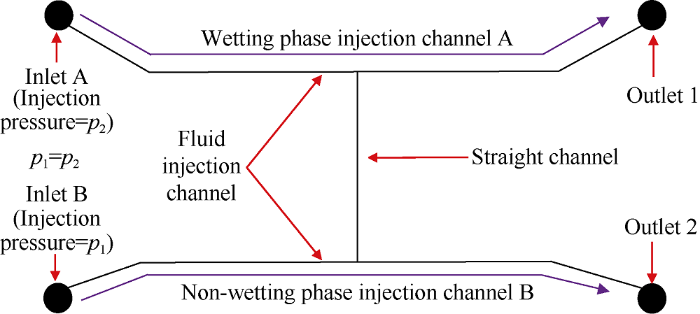
The structure of micromodel used for wettability characterization in this study was shown in Fig. 1, which includes a wetting phase injection channel A (60 mm-length, 0.3 mm- width, 15 μm-depth), a non-wetting phase injection channel B (60 mm-length, 0.3 mm-width, 15 μm-depth), and a thinner straight channel between A and B (30 mm-length, 100 μm-width, 15 μm-depth). The wetting phase and non-wetting phase were injected into the micromodel saturated with non-wetting phase, then the interface of wetting phase and non-wetting phase in the straight channel was observed to measure the two-phase contact angle. Before experiments, the micromodel was soaked in piranha solution (the ratio of concentrated sulfuric acid and hydrogen peroxide was 7:3) for 30 min and then coated with surface modification solution (2.0% trimethylchlorosilane in methanol solution) for 2 h. The oil- water contact angle of the micromodel after soaking was 120°.
Fig. 1.
Fig. 1.
Structure of wettability characterization micromodel.
1.3.3. 2.5D imbibition micromodel
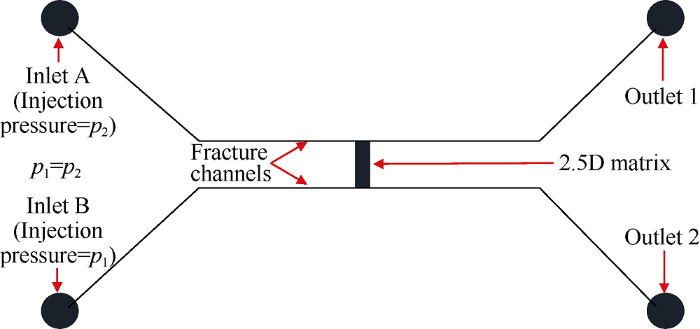
The structure of 2.5D imbibition micromodel used in this study was shown in Fig. 2, including a 2.5D matrix area, two fracture channels, inlets A and B, and two outlets. The two fracture channels are identical in structure and parallel, which are used to provide the contact interface for oil and water during imbibition. When the injection pressure of A and B phase fluids are zero, there is no injection pressure on both sides of the matrix, and the model can simulate imbibition.
Fig. 2.
Fig. 2.
Structure of 2.5D imbibition micromodel.
The fracture channel and the matrix area is connected in T shape to avoid fluid being injected into the matrix area due to the effect of inertia. The fracture channel is 55.5 mm long, 330 μm wide and 16 μm deep. The matrix area is composed of 2.5D pore-throat units with coordination number of 6, is 13 mm long, 3.8 mm wide; and the pore body is about 20 μm deep and the throat is about 4 μm deep (Fig. 3).
Fig. 3.
Fig. 3.
Sturcture of pore throat in 2.5D matrix.
The manufacture method of 2.5D micromodel is proposed by Xu et al.[19], which is based on the isotropic corrosion of HF. A narrower and shallower throat channel between two pore bodies can be created by this method, which is closer to the 3D porous media and has been widely used in oil and gas flow studies[20,21]. The 2.5D imbibition micromodel was also treated by surface modification solution to ensure the glass is completely oil-wet to study the formation and imbibition dynamics of in-situ microemulsion in porous media.
1.4. The procedures
1.4.1. NaCl concentration of micromulsion scan experiment
The procedure of NaCl concentration scan experiment is as follows: (1) The pipette was sintered with alcohol blast burner to seal the bottom outlet. (2) The fixed content of surfactant, co-solvent and water were mixed, and transferred to the pipette with a syringe, then an equal volume of kerosene was added into the pipette, and the initial interface of the oil and water was recorded. (3) Different amounts of NaCl (at the concentration gradient of 0.5%) were added into the pipette, the other end of the pipette was sealed, and the mixture was mixed fully and then set aside. (4) The phase behavior of the microemulsion was observed and the change of interface was recorded.
1.4.2. Wettability characterization experiment
The wetting phase and non-wetting phase were injected into the micromodel saturated with non-wetting phase at a constant pressure of 1.0 kPa. Images of the interface of oil-water phase in the straight channel were recorded when the interface moved.
1.4.3. Microfluidic imbibition experiment
The procedure of microfluidic imbibition experiment is as follows: (1) The kerosene dyed in red was injected into micromodel from inlet A and outlet 1 at a constant pressure of 300 kPa to ensure the micromodel was saturated with kerosene. (2) The kerosene and W Ⅰ were simultaneously injected into the 2.5D imbibition micromodel from inlets A and B at a constant pressure of 2.0 kPa, respectively. When the fluid flow out of the micromodel through outlet, the injection pressure were reduced to 0. (3) The video of flow dynamics in matrix area was recorded in real time. (4) The oil saturation of matrix at different time was calculated by ImageJ software. (5) The micromodel was flushed by 5% hydrochloric acid solution, 2% NaOH solution, ethanol and DI water, respectively after each experiment, and then dried at 120 °C. (6) Each experiment was repeated for 3 times to ensure the accuracy and repeatability of the experiment.
2. Results and discussion
2.1. Salinity scanning
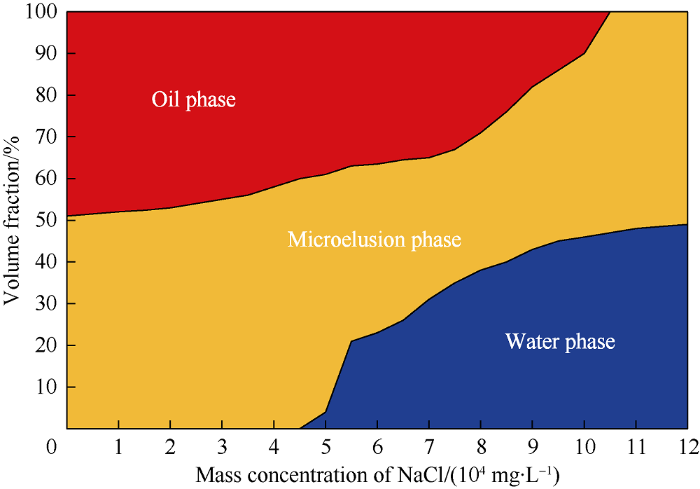
NaCl concentration scanning was conducted to study the static phase behavior of the microemulsion. The soluble oil of the WI system and interfacial tension between the microemulsion and oil at different NaCl concentrations were tested, and the results are shown in Fig. 4. Winsor Ⅰ type (Oil-in-water) microemulsion was observed under low NaCl concentriation. The affinity of surfactant to water and oil can be changed by salting-out effect, which would induce the phase transition of microemulsion from Winsor Ⅰ type to Winsor Ⅱ type and Winsor Ⅱ type with NaCl concentration. In Winsor Ⅰ type microemulsion, the surfactant and cosolvent are more water-wet, and the oil is dispersed in the water in nanodroplets. With the rise of NaCl concentration, the salting-out effect gets stronger, the solubility of surfactant and cosolvent in the NaCl solution drop, and the oil affinity gets stronger, and oil and water would form dual continuous or water-in-oil micro-structure.
Fig. 4.
Fig. 4.
Results of salinity scans.
Based on the phase diagram shown in Fig. 4, the ability to dissolve oil and water (characterized by solubility) can be calculated under different NaCl concentrations in WI system. The solubility of oil or water in unit volume of surfactant is defined by Eq.1.
Adding NaCl into the system can affect the solubility of surfactant and cosolvent, when the NaCl concentration is over 30 000 mg/L, the WI system would stratify under the effect of salt-outing effect, and can’t be used in tertiary recovery. As the soluble oil of WinsorⅠ microemulsion rises almost linearly with the increase of NaCl concentration, therefore, three NaCl concentrations, 0, 10 000, 20 000 mg/L with big gap and lower than the salt resistance limit of WⅠ system were taken to test the effect of NaCl concentration on imbibition efficiency of WⅠ system.
The interfacial tension (IFT) of microemusion and oil was calculated by Chun-Huh equation[22]:
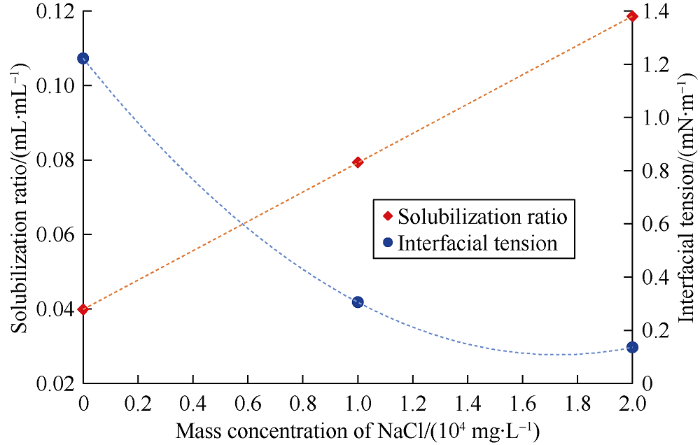
Fig. 5 shows the soluble oil and IFT between oil phase and microemulsion. From the experimental results we can see that the main effect of NaCl concentration is on the soluble oil. In the WⅠ type microemulsion system, the soluble oil increases and the IFT decreases gradually with the increase of NaCl concentration. But the static experiment result can’t reflect the dynamic phase behavior in porous media, the wettability feature and the feasibility of the spontaneous imbibition of the system. More straightforward experiments are needed to provide the information regarding the wetting and spreading behavior of microemulsion.
Fig. 5.
Fig. 5.
Soluble oil and interfacial tension between oil phase and microemulsion variation with NaCl concentration.
2.2. In-situ microemulsion identification and its wetting behavior
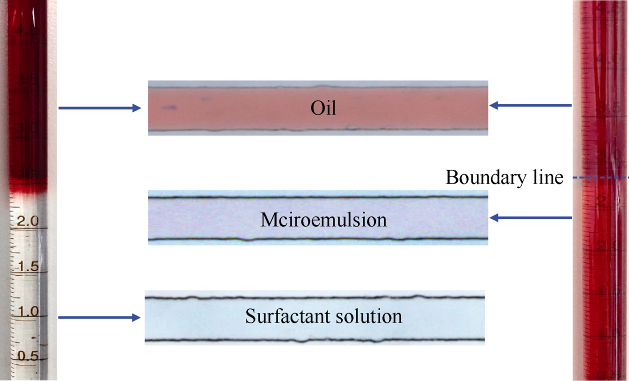
The measurement of contact angle of WⅠ system-oil-solid phase in extra low interfacial tension environment by conventional wetting angle measurement equipment is difficult, because even extremely small drops are flat and miscible immediately in this kind of environment[23]. Whereas the wettability characterization micromodel designed in this study can provide a light disturbance and weak contact condition for aqueous phase and oil phase, which can support the wettability study of microemulsion system. Before wetting behavior experiments, microemulsion system in micromodel was identified first so that the water solution, microemulsion and oil phase can be distinguished in the micromodel experiment (Fig. 6). It is clearly shown that the microemulsion in micromodel is pink, the oil phase is red, and the aqueous phase is transparent.
Fig. 6.
Fig. 6.
Indentification results of microemulsion system in micromodel.
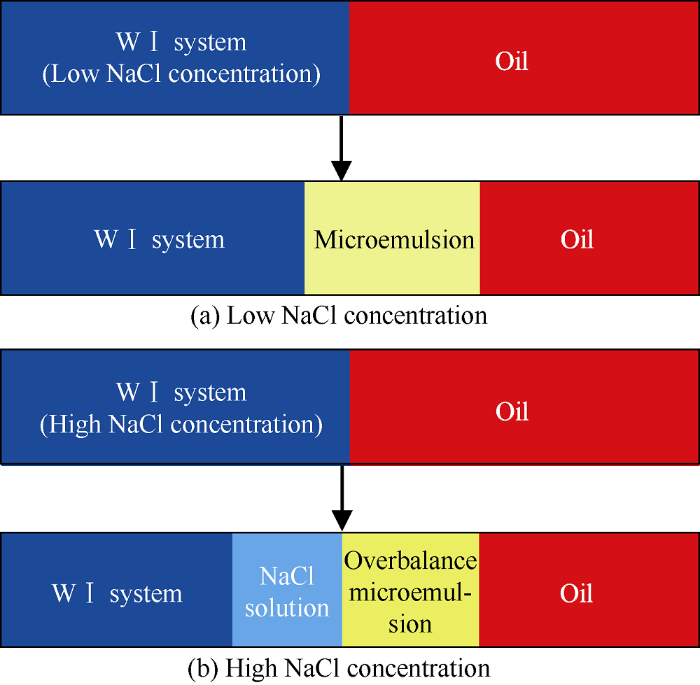
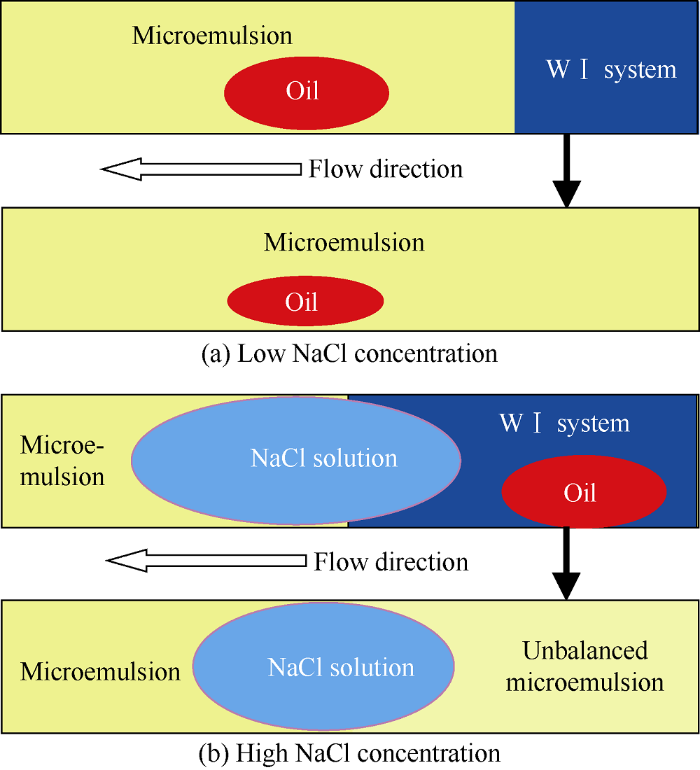
The main purpose of wetting behavior experiments is to provide basis for distinguishing the wetting phase and non-wetting phase. In porous media, oil phase and WⅠ system with low NaCl concentration would form microemulsion under diffusion and convection, as shown in Fig. 7a. According to study results of Tagavifar et al.[21], the dynamic phase change behavior of microemulsion in porous media was different from the static phase change in pipette. The latter conforms to the static equilibrium theory of microemulsion system, while the former conforms to the in-situ local equilib-rium theory of microemulsion system. That is to say the formation of microemulsion and its microstructure are related to local oil to water ratio, local NaCl concentration, and local surfactant concentration under flowing condition. Based on the local equilibrium theory, under the influence of salting out, more surfactant and cosolvent would congregate at the interface of oil and WⅠ system with higher NaCl concentration, forming over-equilibrium microemulsion and NaCl solution would precipitate behind the overbalance microemulsion, as shown in Fig. 7b.
Fig. 7.
Fig. 7.
Schematic diagram of in-situ microemulsion under static and dynamic conditions.
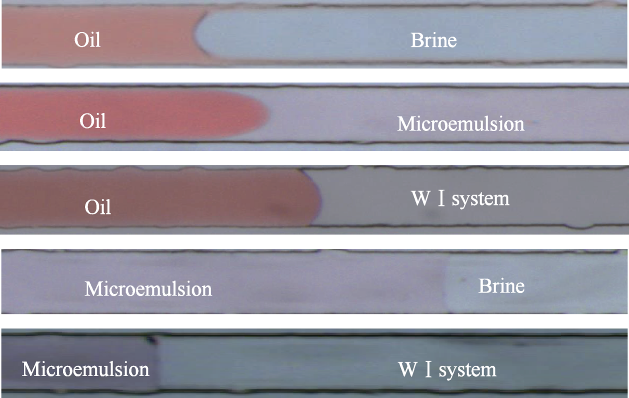
“Overbalance microemulsion” is the emulsion with soluble oil higher than that of the balanced microemulsion caused by local surfactant and cosolvent concentrations higher than those of the original solution. Therefore, in the study of in-situ wettability and spreading of microemulsion, the wetting and spreading behavior of WⅠ system, microemulsion, NaCl solution and oil phase were compared by the wettability characterization micromodel, and the contact angles are shown in Fig. 8. Results show that the order of strength of wettability in oil-wet porous media is WⅠ system or microemulsion, oil phase, NaCl solution, which implies that the WⅠ system and Winsor Ⅰ type microemulsion can induce spontaneous imbibition in oil-wet reservoirs.
Fig. 8.
Fig. 8.
Contact angles between oil, NaCl soluition, WⅠ system and microemulsion in oil-wet micmodel.
2.3. Influence of NaCl concentration on WⅠ system imbibition
2.3.1. Imbibition efficiency
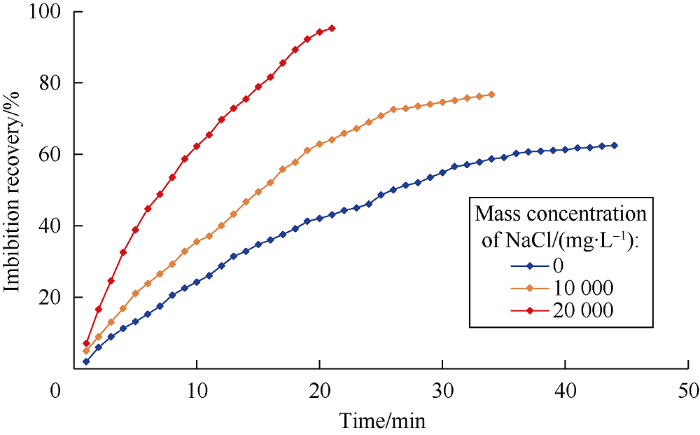
Imbibition experiments were conducted in oil-wet 2.5D micromodels, and the results were shown in Fig. 9. It can be seen clearly that, the imbibition recovery of WⅠ system with the NaCl concentration of 0, 10 000, 20 000 mg/L are 62.5%, 76.8%, 95.3%, respectively, which implies that the imbibition rate and ultimate recovery of oil increase with the increase of NaCl concentration, which means the WⅠsystem could improve spontaneous imbibition efficiency of oil-wet medium .
Fig. 9.
Fig. 9.
Imbibition effect of WⅠ system at different NaCl concentrations.
From the imbibition process in Fig. 10, it can be seen that, (1) when the NaCl concentration was 0 mg/L, only oil phase and Winsor Ⅰ type microemulsion were observed in the micromodel; (2) With the increase of NaCl concentration, the area of microemulsion became narrower, which implies the Winsor Ⅰ type microemulsion formed could not solubilize into the following surfactant instantly; (3) With the increase of NaCl concentration, the imbibition rate became faster, more oil droplets trapped in the swept area and in the early stage of imbibition would be recovered, and this would be obvious when the NaCl concentration was 20 000 mg/L.
2.3.2. Imbibition mode
Based on the results in Fig. 10, the imbibition mode of Winsor Ⅰ type surfactant solutions can be divided into two kinds:
Fig. 10.
Fig. 10.
Imbibition process of three kinds of surfactant soluitons with different NaCl concentrations.
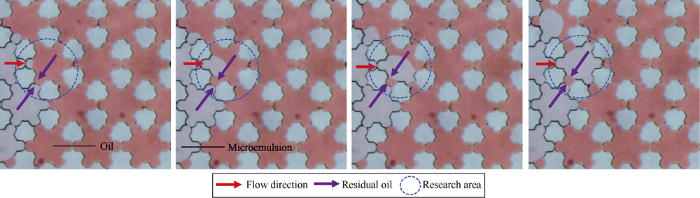
(1) Under lower NaCl concentration, with weak salting-out effect, surfactant and cosolvent have stronger affinity for water phase, the microemulsion formed at the contact interface of oil phase and WⅠ system can be miscible with subsequent WⅠ system constantly, and Winsor Ⅰ type microemulsion would reach equilibrium state immediately as shown in Fig. 10a. Hence, only oil phase and microemulsion phase can be observed at low NaCl concentration. The whole imbibition process can be simplified as the driving of the non-wetting phase (oil phase) by the wetting phase (microemulsion) (Fig. 11). In this process, there existing contact between oil and microemulsion in several throats, after the breakthrough of microemulsion in one throat, the imbibition residual oil would be formed under the capillary shielding effects, as shown in Fig. 11 which is indicated by the purple arrows.
Fig. 11.
Fig. 11.
Micorscopic images of microemulsion imbibition at the NaCl concentration of 0 mg/L.
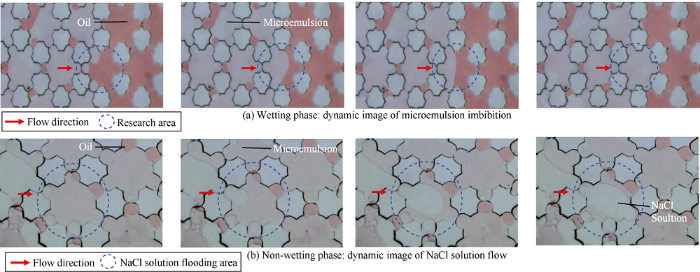
(2) At higher NaCl concentration, with stronger salting-out effect, surfactant and cosolvent have relatively stronger affinity to oil pahse, more surfactant and cosolvent would congregate at the contact interface of oil and WⅠ system, inducing the formation of microemulsion and NaCl solution. At this point, WⅠ system, microemulsion, NaCl solution and oil phase can be observed in the oil-wet porous media, as shown in Fig. 10b and Fig. 10c. In the process of imbibition, the microemulsion would spread in wall surface as wetting phase, which is shown in Fig. 12a. The NaCl solution would displace the microemulsion phase under the driving of following WⅠ system, as shown in Fig. 12b.
Fig. 12.
Fig. 12.
Micorscopic images of WⅠ system imbibition at the NaCl concentration of 20 000 mg/L.
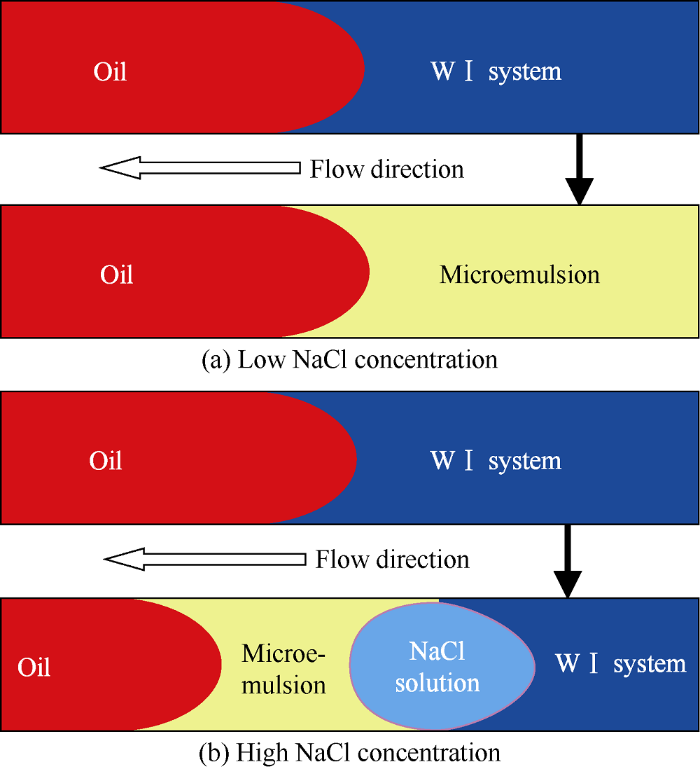
Based on the above description, the imbibition mode of WⅠ system can be characterized as microemulsion-oil imbibition at lower NaCl concentration and WⅠ system-NaCl solution-microemulsion-oil imbibition at higher NaCl concentration, which can be explained by Fig. 13.
Fig. 13.
Fig. 13.
The influence of NaCl concentration on imbibition mode of in-situ microemulsion system.
2.3.3. Solubilization efficiency
Fig. 14 shows the recovery process of residual oil trapped in the swept areas, red arrows indicate the flow direction of the following WⅠ system. It can be seen that the boundary of WⅠ solution and microemulsion becomes fuzzy, which implies the solubilization of microemulsion and WⅠ system. After mutual dissolution, the unbalanced microemulsion transformed into balanced microemulsion (blue dotted areas) which is capable of solubilizing residual oil. It also can be seen that this solubilization effect is local, residual oil doesn’t involve in rebalancing would not be solubilized.
Fig. 14.
Fig. 14.
Flow dynamic of following WⅠ system.
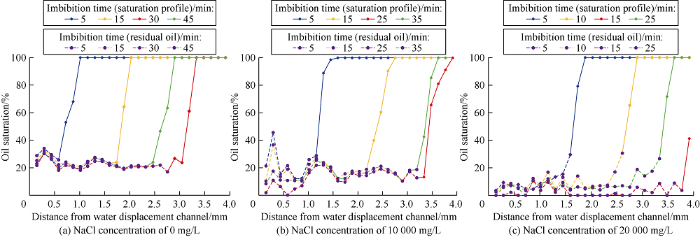
The oil saturation profiles of the micromodel were calculated by imageJ software, to quantify the recovery of trapped oil in WⅠ system during the imbibition process, as shown in Fig. 15. The dotted lines indicate the oil saturation of swept areas. It can be seen that, (1) for the WⅠ system with NaCl concentration of 0 mg/L, the residual oil saturation after imbibition is around 20%, and the trapped oil in the swept area is hardly recovered by the following WⅠ system; (2) For the WⅠ system with NaCl concentration of 10 000 mg/L, the residual oil saturation after imbibition is around 15%, and the residual oil at the end of the model can be recovered by the following WⅠ system due to its solubilization; (3) For the WⅠ system with NaCl concentration of 20 000 mg/L, the residual oil saturation after imbibition is around 10%, and most of trapped oil in the swept area can be recovered by the following WⅠ system.
Fig. 15.
Fig. 15.
Oil saturation and residual oil saturation profiles of different WⅠ systems.
Based on the results in Figs. 5 and 15, we can conclude that: (1) the residual oil saturation is negatively correlated with the NaCl concentration in WⅠ system; (2) The recovery of residual oil is positively correlated with the NaCl concentration of WⅠ system. In 2.5D imbibition model, the WⅠ system is the excess phase, which implies the solubilization volume of oil is much more than the volume of residual oil under all the three conditions. Therefore, the recovery of residual oil isn’t strongly correlated with the soluble oil volume in WⅠ system. Different rebalancing mechanism under different NaCl concentrations induced different residual oil recovery effects. WⅠ system, NaCl solution with high concentration, microemulsion and oil coexist in the porous media under the flow condition, the surfactant would gather at the interface of NaCl solution, forming an interface film with certain strength, and an isolating belt would be formed near the interface film between WⅠ solution and Winsor Ⅰ type microemulsion, which would block the fast solubilization of the water soluble Winsor Ⅰ type microemulsion and following WI system, and make the following WⅠ system dissolve more oil (Fig. 16).
Fig. 16.
Fig. 16.
Effect of NaCl concentration on residual oil solubilization and imbibition in WⅠ system.
3. Conclusions
The Winsor Ⅰ type surfactant solution and WⅠ system are wetting phase compared to NaCl solution and oil phase in oil-wet porous media. The NaCl solution produced by salting-out effect is the non-wetting phase compared to oil.
The Winsor Ⅰ type surfactant solution would form in-situ microemulsion with oil, and its main mechanisms of enhancing imbibition recovery are wetting and spreading and residual oil solubilization.
The imbibition mode of WⅠ system can be strongly affected by NaCl concentration, which can be characterized as Winsor Ⅰ type microemulsion-oil imbibition at lower NaCl concentrations and WⅠ system - NaCl solution - Winsor Ⅰ type microemulsion - oil imbibition at higher NaCl concentrations. The latter has higher imbibition efficiency.
The solubilization efficiency of WⅠ system for residual oil can be also affected by NaCl concentration: the Winsor Ⅰ type microemulsion can be solubilized with following WⅠ solution instantly under low NaCl concentration, forming unbalanced WⅠ microemulsion with poorer solution to residual oil. At higher NaCl concentration, the solution of Winsor Ⅰ microemulsion with NaCl solution is slower, the following WI system would form Winsor Ⅰ type microemulsion with residual oil, to produce more oil.
Nomenclature
C— constant, with a value of 0.3 mN/m;
Vs— volume of surfactant, mL;
Vx—volume of oil or water solubilized in microemulsion, mL;
γ—interfacial tension between microemulsion and oil, mN/m;
σx—solubility of oil or water in unit volume of surfactant, mL/mL.
Reference
Breakthrough and significance of unconventional oil and gas to classical petroleum geological theory
Geological conditions for continental tight oil formation and the main controlling factors for the enrichment: A case of Chang 7 Member, Triassic Yanchang Formation, Ordos Basin, NW China
A numerical simulation model for multi-scale flow in tight oil reservoirs
Profitable exploration and development of continental tight oil in China
Evaluation of liquid nanofluid as fracturing fluid additive on enhanced oil recovery from low-permeability reservoirs
Nuclear magnetic resonance features of low-permeability reservoirs with complex wettability
What type of surfactants should be used to enhance spontaneous imbibition in shale and tight reservoirs?
Application of nanotechnology in petroleum exploration and development
Experimental investigation of CO2, huff-n-puff process for enhancing oil recovery in tight reservoirs
Experimental study on spontaneous imbibition of recycled fracturing flow-back fluid to enhance oil recovery in low permeability sandstone reservoirs
Review of ASP EOR (alkaline surfactant polymer enhanced oil recovery) technology in the petroleum industry: Prospects and challenges
Evaluation of wettability alteration and IFT reduction on mitigating water blocking for low-permeability oil-wet rocks after hydraulic fracturing
Flow physics of how surfactants can reduce water blocking caused by hydraulic fracturing in low permeability reservoirs
Scaling of low-interfacial-tension imbibition in oil-wet carbonates
Experimental investigation of imbibitions in oil-wet carbonates under low IFT conditions
Favorable attributes of alkali-surfactant-polymer flooding
A systematic laboratory approach to low-cost, high-performance chemical flooding
A 2.5-D glass micromodel for investigation of multi-phase flow in porous media
Egalitarianism among bubbles in porous media: An Ostwald ripening derived anticoarsening phenomenon
Spontaneous and flow-driven interfacial phase change: Dynamics of microemulsion formation at the pore scale
Equilibrium of a microemulsion that coexists with oil or brine
Contact angles for equilibrated microemulsion systems