Introduction
Mature oilfields often have considerable remaining reserves, and remain to be the main force of oil development for some time to come[1]. Chemical flooding has become a common technique to enhance oil recovery for mature oilfields, and has achieved good development effects[2]. At present, there are many kinds of chemical agents used for chemical flooding. Chemical agents with single function still have different degrees of drawbacks, and are limited in adaptability. The synergy effect of different chemical agents with different functions in compound system is difficult to play[3]. Surface-active polymer is a novel active multifunctional polymer with the functions and properties of both polymer and surfactant, which can meet the requirements of enhancing oil recovery in mature oilfields[4].
Researches, experiments and applications of surface-active polymer have undergone for more than 10 years in China. Previous studies focused on the evaluation of performance and application effect of surface-active polymer, the viscosity property and flooding effects of surface-active polymers have been extensively tested[5,6], and some basic understandings on the physical and chemical properties, flow characteristics and oil recovery enhancement effect of the surface-active polymers have been obtained. But the mechanism of enhanced oil recovery by surface-active polymer hasn’t been studied completely, and needs to be studied in-depth and systematically.
In this study, experiments on surface-active polymer flooding in Daqing placanticline oilfield were carried out by using detection analysis and modern physical simulation techniques under the guidance of physical, chemical and reservoir engineering theories, to find out the oil recovery enhancing mechanisms and develop basic theory of surface-active polymer, in the hope to provide technical support for enhanced oil recovery of mature oilfields.
1. Properties and characteristics of surface-active polymer
Properties and characteristics of surface-active polymer are the main factors controlling its oil recovery performance, so to make clear the properties of surface-active polymer and the differences of surface-active polymer from other chemical agents is the precondition to sort out the oil recovery enhancing mechanisms of surface-active polymer.
1.1. Molecular composition and structure of surface-active polymer
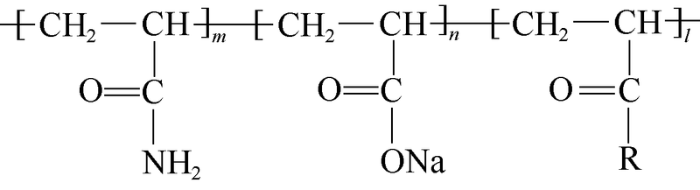
Surface-active polymer is formed by grafting and copolymerization of multiple functional groups using the hydrocarbon chain of acrylamide and sodium acrylate as skeleton. The introduced functional groups are dendritic hydrophobic monomer, alkyl sulfonate surfactant unit, non-ionic active sulfides unit, cationic quaternary ammonium Gemini surfactant unit, and cationic quaternary ammonium salt surfactant unit (Fig. 1). The surface-active polymer specially modified for Daqing placanticline oilfield is developed and modified based on properties of the reservoirs and fluids in Daqing placanticline oilfield. It is different from other surface-active polymers in type, location and quantity of active functional groups and relative molecular mass size.
Fig. 1.
Molecular structure diagram of surface-active polymer. R for —OR, —NHR, —RSH, —(EO)n(PO)mR, —RSO3Na or other functional groups.
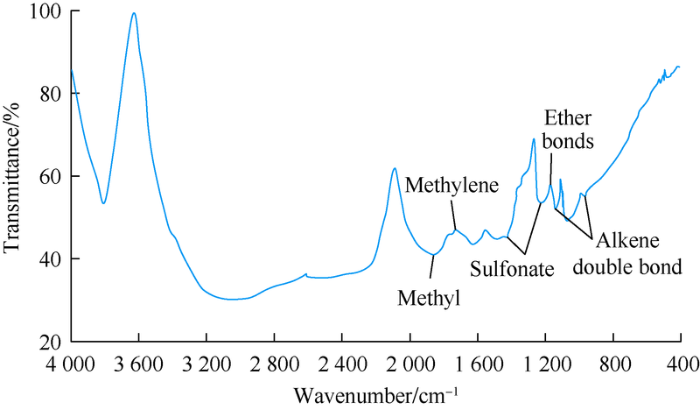
Molecular structure and characteristic functional group of surface-active polymer can be characterized by infrared spectrum (Fig. 2). The surface-active polymer has an amphiphilic structure, as characteristic absorption peaks of sulfonate, ether bonds, methyl and methylene appearing in infrared spectrum indicate the presence of hydrophilic groups and lipophilic groups in molecular structure of the surface-active polymer. The characteristic absorption peak of alkene double bond caused by residual monomer shows that polymerization effect occurs in the synjournal process of the surface-active polymer.
Fig. 2.
Infrared spectrum of surface-active polymer.
1.2. Basic physicochemical properties of surface-active polymer
The surface-active polymer has good dissolution performance, fast dissolution rate at early stage, then slower after reaching certain hydrolysis degree, and hydrolysis degree of 21.5% at equilibrium state. The surface-active polymer solution can be maintained at high viscosity, and the surface-active polymer solution with a mass concentration of 800 mg/L has a viscosity of greater than 42.3 mPa·s after stabilization (Table 1).
Table 1 Physicochemical properties of the surface-active polymer.
| Parameter | Value | Parameter | Value | |
|---|---|---|---|---|
| Density | 0.653 g/cm3 | Viscosity | ≥42.3 mPa·s | |
| Solid content | 88.83% | Water insoluble content | 0.07% | |
| Hydrolysis degree | 21.5% | Dissolution rate | ≤1 h |
1.3. Differences between the surface-active polymer and other chemical flooding agents
Different molecular composition and structure make the properties and characteristics of surface-active polymer very different from those of ordinary polymers. The surface-active polymer has functions and properties both of polymer and surfactant, but there are great differences between surface-active polymer and the binary compound system of polymer and surfactant.
Experiments were carried out to test the property differences between surface-active polymer and ordinary polymer and polymer-surfactant binary compound system. The chemical agents in the experiment include the surface-active polymer for Daqing placanticline oilfield with relative molecular mass of 1250×104, partial hydrolyzed polyacrylamide (HPAM) representing ordinary polymer with a relative molecular weight of 1300×104, and partial hydrolyzed polyacrylamide with a relative molecular weight of 1300×104 and heavy alkyl benzene sulfonate surfactant with mass fraction of 0.3% (HPAM/HABS) representing binary compound system. The oil used in the experiment was prepared by mixing degassed crude and kerosene at certain ratios (usually 1:3 to 1:9), which has a viscosity of 9.8 mPa·s at formation temperature. The water used in the experiment has a salinity of 3700 mg/L and composition and content of mineral ions same as the injection water in Daqing placanticline oilfield (typical mature oilfield with extremely high water-cut). The main test instruments include HITACHI S-3400N scanning electron microscope, BROOKHAVEN BI-200SM dynamic/static laser scattering apparatus, BROOKFIELD DV-II+PRO Brookfield viscometer, HJ-6 magnetic stirrer, thermostat, electronic balance, beaker and test tube etc. The displacement experimental apparatuses include constant-flux pumps, pressure sensors, core holders, hand pumps and intermediate containers.
1.3.1. Features of molecular aggregation
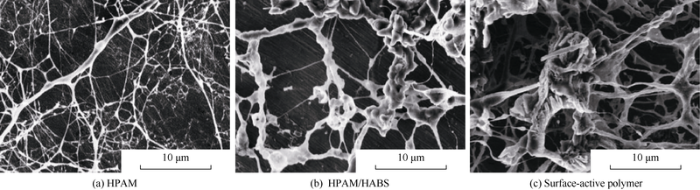
SEM pictures show the ordinary polymer, binary compound system and surface-active polymer differ widely in molecule aggregation conformation (Fig. 3).
Fig. 3.
SEM pictures of molecule aggregation conformation of different chemical agents.
(1) The ordinary polymer molecules of HPAM appear as long-chains entangling with each other to form irregular cross-linking net. Strong electrostatic repulsion causes the molecular chains to stretch, forming the backbone and branches of the cross-linking structure. The chains are evenly distributed and about 0.06-1.30 μm in coil size. (2) Ions of HABS in the binary compound system compress polymer molecular coils, and make the space skeleton sparse; increased intermolecular thermal motion make the molecules increase in hydrodynamic diameter, so the molecular coils in the compound system are larger (0.06-3.50 μm). (3) Molecules of surface-active polymer are stouter than HPAM and HPAM/HABS and appear in small aggregates because of the stronger association and cross-linking effect between molecules, so the aggregates come in regional network. The molecular chains of surface-active polymer range widely and with coil size of 1.7 μm to 5.7 μm.
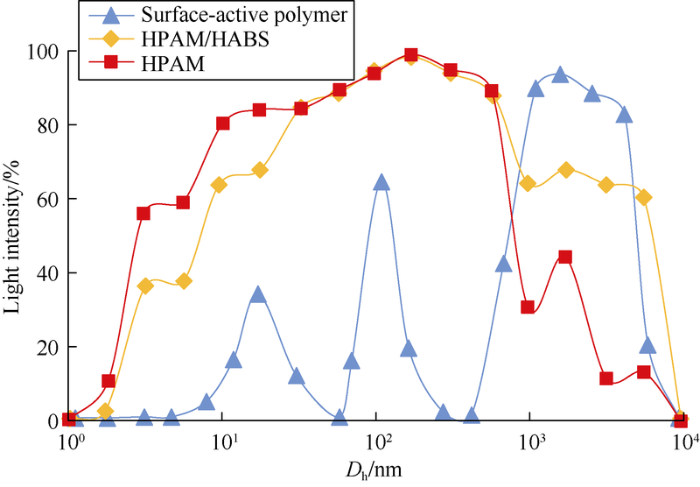
Surface-active polymer, binary compound system and polymer at the same mass concentration were tested by dynamic/static laser scattering apparatus to compare the dimension of their molecular coil (Dh) (Fig. 4). The Dh value of surface-active polymer is the largest, followed by the binary compound system, and the Dh value of polymer is the smallest. The difference between the Dh values of binary compound system and polymer is small, but the Dh value of polymer is far less than that of surface-active polymer. The distribution of the Dh value of polymer is the most concentrated, and the distribution of Dh value of surface-active polymer is the most dispersed. Crosslinking reaction occurs in and between molecules of surface-active polymer solution, which make molecular coil size the largest.
Fig. 4.
Dh distribution curves of the chemical agents.
1.3.2. Performance of viscosity
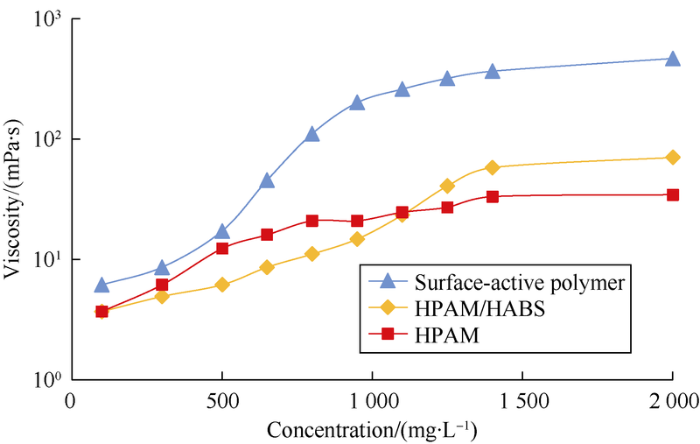
Surface-active polymer has stronger viscosity increasing effect than binary compound system and polymer (Fig. 5). Under the same mass concentration, the surface-active polymer has the largest viscosity, as the molecular structure of branch and crosslinking caused by the introduction of surfactant groups makes the viscosity increase. The viscosity of polymer is minimal. Surfactants in binary compound system enable the production of micelles and formation of complexes of polymer and surfactant, in turn increase of the hydrodynamic volume and slight increase of its viscosity. In addition, all the three agents increase in viscosity with the increase of concentration, and the surface-active polymer has the highest amplitude of viscosity increase.
Fig. 5.
Viscosity-concentration relationship of the chemical agents.
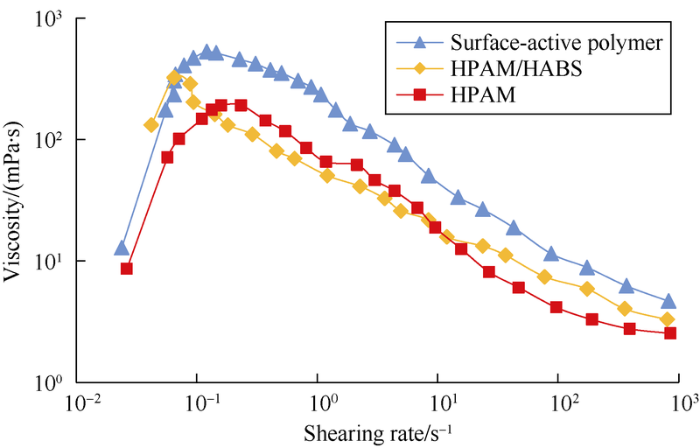
The test of effect of shearing rate on viscosity shows when shearing rate is in a low range (less than 0.1 s-1), the viscosity of surface-active polymer, HPAM and HPAM/HABS increase with the increase of shearing rate (Fig. 6); beyond this range, the viscosity decreases with the increase of the shearing rate. The rheological behavior of shear-thinning and shear-thickening of surface-active polymer are more pronounced, as the particle cluster of surface-active polymer is large during the shearing process and the network structure of surface-active polymer has poor stability.
Fig. 6.
Relationship between viscosity of chemical agent and shearing rate.
1.3.3. Capability of flow
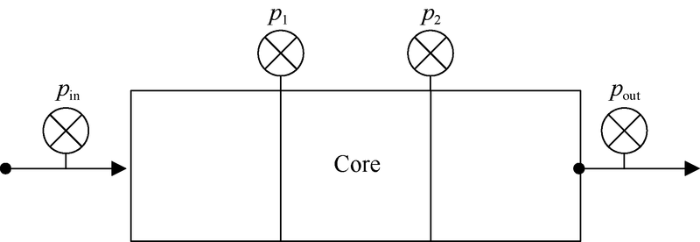
Displacement experiments were carried out using artificial cores of the same permeability and solutions of chemical agents with the same concentration. The core used in the experiment was made by quartz cemented by epoxy resin in rectangular shape, and was 45 mm×45 mm×300 mm, with gas permeability of 1000×10-3 μm2. The mass concentration of the solution used in the experiment was 1000 mg/L. The transmission and flow capacity model as shown in Fig. 7 was established. The solution was injected at a constant rate of 0.3 mL/min during the experiment, and the pressures at the inlet, trisection and outlet of the core were measured to compare the pressure variations at different locations of the core at the end of the flooding and evaluate the transmission capacity.
Fig. 7.
Schematic diagram of experimental model for transmission and flow capability.
The experimental results show that the pressure differences between the front, middle and rear parts of the core after ordinary polymer flooding are small, that means the ordinary polymer solution has strong transmission capability, and only a small amount of ordinary polymer is evenly retained in the core (Table 2). The pressure differences between the three parts of the core after surface-active polymer flooding are much higher, the pressure drop of the first one-third part is much larger than the other parts, and the retained amount is the largest and there is even clog at the inlet end of the core, which indicate that the surface-active polymer solution has poorer transmission capability. The pressure differences in the front and middle parts of the core after binary compound system flooding are lower than those after surface-active polymer flooding, but larger than those after polymer flooding, so the transmission capability of binary compound system is between that of polymer solution and surface-active polymer solution.
Table 2 Pressure differences in different parts of the core.
| Chemical agents | Pressure differences in different parts/MPa | ||
|---|---|---|---|
| Front(p1-pin) | Central(p2-p1) | Rear (pout-p2) | |
| Surface-active polymer | 0.361 | 0.038 | 0.001 |
| Binary compound system | 0.057 | 0.021 | 0.015 |
| Polymer | 0.033 | 0.019 | 0.017 |
As the molecular chains of ordinary polymer solution have stronger flexibility, most HPAM molecules captured by pores and throats can be flushed out during subsequent water flooding, leading to the decrease of flow resistance, so resistance factor and residual resistance factor of ordinary polymer solution are both the lowest (Table 3). With big aggregates of molecules, the surface-active polymer solution even clogged the injection surface of the core, leading to higher injection pressure, so the resistance factor of surface-active polymer solution is the largest. The flow resistance of binary compound system is slightly higher than that of polymer solution and much lower than that of surface-active polymer solution, showing flow capability in between polymer solution and surface-active polymer solution.
Table 3 Experimental results of resistance factor and residual resistance factor.
| Chemical agents | Resistance factor | Residual resistance factor |
|---|---|---|
| Surface-active polymer | 303.5 | 117.1 |
| Binary compound system | 33.9 | 15.5 |
| Polymer | 20.3 | 6.7 |
2. Mechanisms of increasing oil-washing efficiency by surface-active polymer flooding
Surface-active polymer is chemical flooding agent that combines the functions of polymer and surfactant, and surface-active polymer solution enables the combination of viscosity and activity in low relative molecular weight and low concentration water solution, to give full play to the positive effects of viscoelastic property and improve chemical properties of the interface and oil-washing efficiency.
2.1. Positive effects of viscosifying and viscoelastic properties
Surface-active polymer solution works well in increasing viscosity. The interface viscosity of surface-active polymer and oil is much greater than the interface viscosity of water and oil, so the shearing stress of surface-active polymer and oil is much greater than the shearing stress of water and oil. Surface-active polymer solution has the ability to carry oil because of the tensile force on oil film produced in the shearing direction[7]. When flowing through porous media, the viscoelastic surface-active polymer solution would produce a normal stress perpendicular to the displacement direction, so surface-active polymer solution has the ability to drive the oil behind and on the sides to flow[8,9]. The oil carrying and pulling ability of surface-active polymer work jointly (Fig. 8) to get the residual oil originally unmovable to move, and this is one of the mechanisms of surface-active polymer to enhance oil washing efficiency.
Fig. 8.
Positive effects of viscosity and viscoelastic properties.
The viscoelastic property of modified surface-active polymer used in Daqing placanticline oilfield was tested with the same instrument used to test its properties.
2.1.1. Viscosity-increasing performance
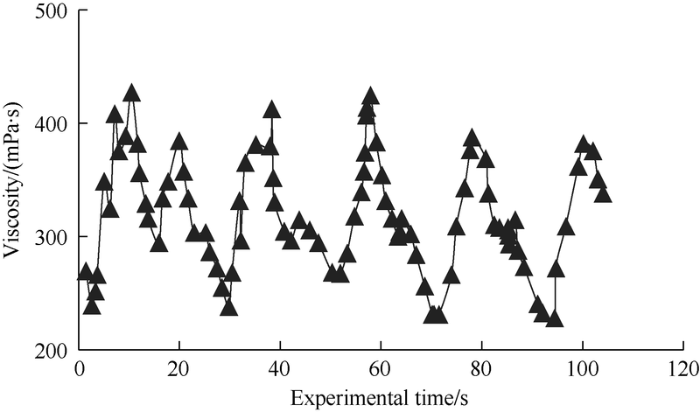
The test results of viscosity, viscosity stability, shearing resistance and salinity resistance show that the surface-active polymer solution has good viscosity-increasing performance and viscosity maintaining ability. The test results (Fig. 9) show that the surface-active polymer can increase viscosity significantly, and has higher dynamic viscosity. The molecules of surface-active polymer have strong self-crosslinking ability, and are likely to form independent molecular aggregates, making the apparent viscosity of surface-active polymer solution fluctuate greatly and regularly.
Fig. 9.
Dynamic viscosity variation curve of surface-active polymer solution.
Surface-active polymer has strong antioxidant ability because of effective decomposition of peroxides by self-oxidation of easily oxidized functional groups in molecular chain. The stability test results show surface-active polymer solution changes little in viscosity after 100 d from the initial viscosity (Fig. 10).
Fig. 10.
Viscosity stability curve of surface-active polymer solution.
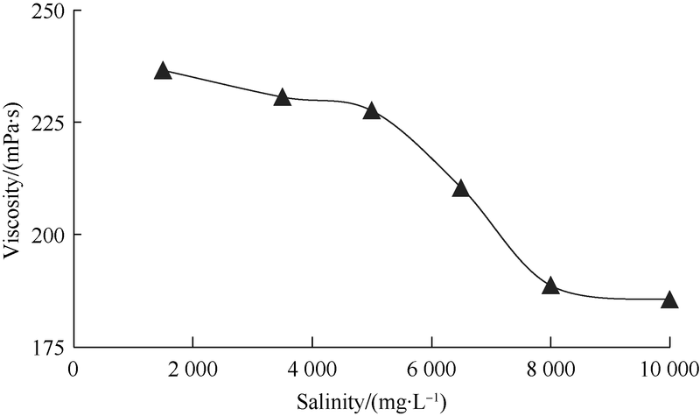
As the minerals in formation water would affect surface- active polymer molecules, the salinity increases, the repulsion between molecules would decrease, the molecular chains would become curly, and the viscosity of surface-active polymer solution would decrease (Fig. 11). The test shows at the tested salinity of up to 10 000 mg/L, the viscosity of the surface-active polymer can be kept at a retention rate of over 75%, so the surface-active polymer has quite good tolerance to salt.
Fig. 11.
Relationship between viscosity and salinity of surface-active polymer solution.
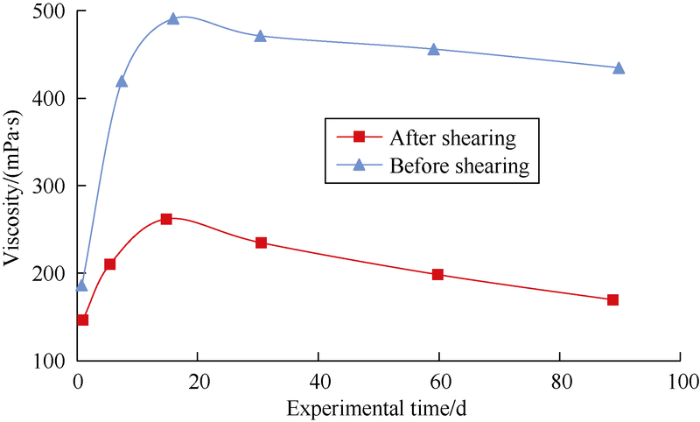
The viscosity retention rates of surface-active polymer solution before and after shearing 90 d were tested and compared (Fig. 12). The results show that the retention rates of viscosity before and after shearing are both greater than 100%, that means the surface-active polymer solution after shearing still has the ability of viscosity restoration.
Fig. 12.
Viscosity variation curve of surface-active polymer solution with time before and after shearing.
2.1.2. Viscoelastic properties
Results of steady-state shearing and dynamic mechanical experiments show that the surface-active polymer solution has good viscoelasticity and deformability. From the steady-state shearing experiment (Fig. 6), when shearing rate is less than 0.1 s-1, the viscosity of the surface-active polymer increases with increase of shearing rate; when shearing rate is more than 0.1 s-1, the viscosity decreases with the increase of shearing rate.
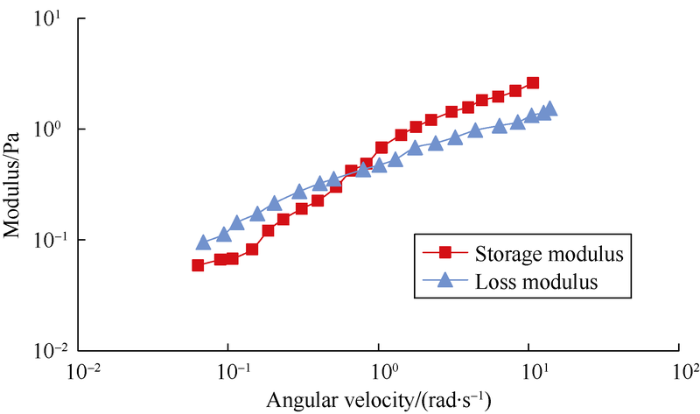
The results of dynamic mechanical experiments show the storage modulus (G′) and loss modulus (G″) of surface-active polymer increase with the increase of angular velocity in small amplitude oscillatory shearing (Fig. 13). Storage modulus indicates the storage of energy in the process of fluid deformation, which reflects the elastic property of the fluid, and loss modulus indicates the consumption of energy in the process of fluid deformation, which reflects the viscous property of the fluid. When the angular velocity is within a relatively low range, G″ is greater than G′, and the surface-active polymer solution has greater viscous property than elastic property, and mainly shows viscous flow. When the angular velocity is greater than a certain value, G′ is greater than G″, the surface-active polymer solution has greater elastic property than viscous property, and shows mainly elastic flow.
Fig. 13.
Relationship between modulus of surface-active polymer solution with angular velocity.
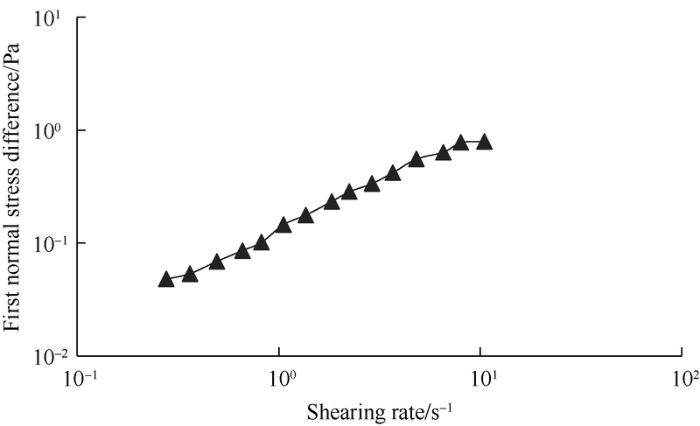
The first normal stress difference is one of the main features of viscoelastic fluid, and the change of first normal stress difference with shearing rate reflect the elasticity of fluid. The first normal stress difference of surface-active polymer solution tends to rise linearly with shearing rate (Fig. 14).
Fig. 14.
Relationship of first normal stress difference of surface-active polymer solution with shearing rate.
2.2. Improvements of interfacial chemical properties
The interfacial chemical properties in the displacement process refer to the physicochemical characteristics of the interface system of displacing phase and displaced phase, and the change of interfacial chemical properties affect the physical and chemical law and processes during the displacement. Surface-active polymer can improve the interfacial chemical properties of oil and water, and then improve microscopic oil-washing efficiency. The interfacial tension and wettability of the surface-active polymer solution used in Daqing placanticline oilfield were tested with TX550A spinning drop interfacial tension meter and OCA20 optical interfacial contact angle meter.
2.2.1. Reduction of interfacial tension
Different polar groups in the surface-active polymer tend to dissolve in similar polar solutions, the polar difference on the oil-water interface decreases, the interface energy and the interfacial tension decrease, and oil adsorbed on the rock surface is more likely to dissolve in the displacing phase, thus improving microscopic oil-washing efficiency[10].
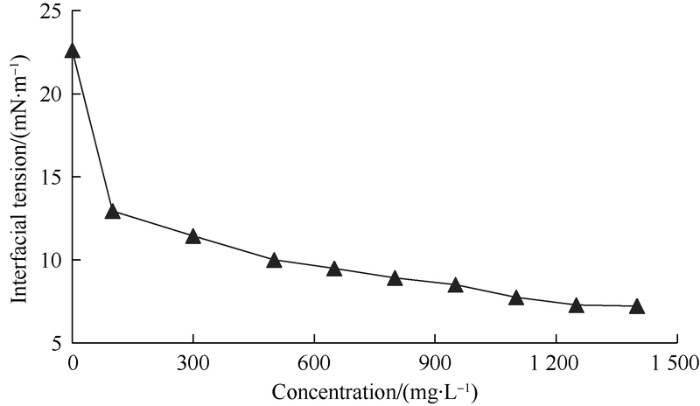
The results of interfacial tension test of surface-active polymer solutions with different concentrations (Fig. 15) show that with the increase of mass concentration of surface- active polymer, the interfacial tension of produced liquid from surface-active polymer flooding reduces rapidly at first and then slower. But even surface-active polymer solution with high concentration can’t reach ultra-low interfacial tension, therefore, reducing interfacial tension isn’t the main mechanism of enhancing oil recovery of the surface-active polymer.
Fig. 15.
Interfacial tension of surface-active polymer solutions with different concentrations.
2.2.2. Emulsification of crude oil
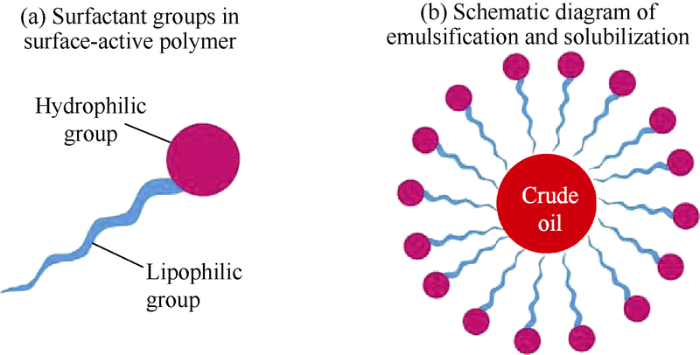
During the flooding of surface-active polymer, the surface-active polymer molecules adsorb on the oil-water interface directionally, the drop of interfacial tension makes the oil disperse in the water more easily, the water and deformed residual oil contacting surface-active polymer would form stable oil-water emulsion under shearing[11]. The dissolution during emulsion has the effect of solubilization (Fig. 16). The surface-active polymer changes the original oil/water interface film into surface-active polymer/oil interface film, so the elasticity of the interface film increases and the adhesion of the residual oil on the pore wall weakens, making it easier for the residual oil to deform, fall off and emulsify. Meanwhile, the interface film makes the possibility of residual oil re-adsorption on to the rock reduce and the emulsion stable to some extent. Under non-ultralow interfacial tension, the emulsion capacity of surface-active polymer is an important characteristic to enhance oil-washing efficiency. In the past, ultra-low interfacial tension was pursued to improve displacement efficiency in most of the chemical flooding projects, and the emulsification of crude oil by chemical agents was not paid enough attention.
Fig. 16.
Schematic diagram of emulsification and solubilization performance of surface-active polymer.
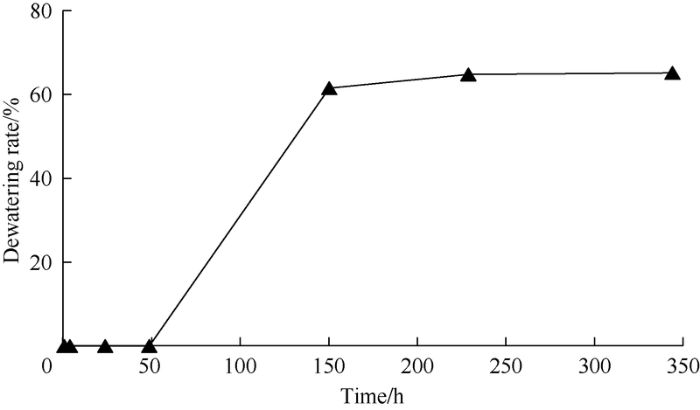
The emulsion was prepared with crude oil and surface-active polymer solution at 1:1, the interface variation of oil and water and water separated out of the emulsion were observed after standing for some time, and the emulsifying ability of the surface-active polymer was analyzed by using the beginning time of water separation and water separating ratio. The longer it takes for the water to separate, and the lower the water separating ratio, the higher the stability of the emulsion and the stronger the emulsifying ability of the surface-active polymer will be. The test results (Fig. 17) show that the beginning time of water separation is about 48 h, the state can remain emulsified after standing for 6 d, and the final maximum water separating ratio is 63%, proving the surface-active polymer has strong emulsifying ability.
Fig. 17.
Dewatering rate of emulsion generated by surface-active polymer with time.
2.2.3. Change of wettability
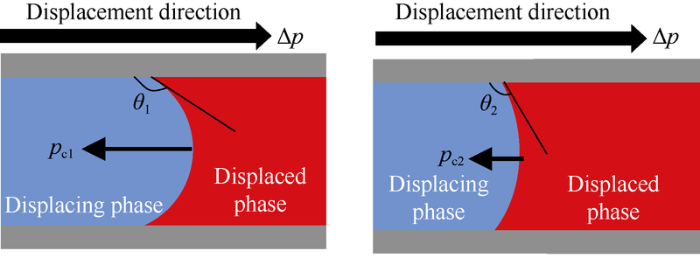
Wettability of reservoir rock affects capillary pressure and relative permeability. When the reservoir rock turns to water-wet, the capillary pressure would drop even turn around to the displacement direction, and thus the displacement resistance would reduce (Fig. 18). Moreover, when the rock turns to water-wet, the contact angle between crude oil and rock surface would decrease, and the adhesion would drop, and the oil and water relative permeability would turn the direction favorable for oil flow.
Fig. 18.
Schematic diagram of rock wettability to water-wet.
The contact angle of reservoir rock decreased after surface-active polymer flooding, the reduction amplitude increased with the increase of concentration of surface-active polymer, and the rock wettability had the trend of turning water-wet (Fig. 19). The introduction of surfactant makes the surface-active polymer adhere to the reservoir rock surface, and thus makes the wettability turn from oil-wet to water-wet. Under non-ultralow interfacial tension, the change of rock wettability to water-wet has some effect on improving oil-washing efficiency[12].
Fig. 19.
Contact angles before and after flooding with surface-active polymer solutions with different concentrations.
3. Performance of enlarging swept volume by surface-active polymer flooding
The surface-active polymer can increase the swept volume, which is an important recovery enhancing mechanism. The mobility control effect of the surface-active polymer was tested first to analyze the mechanism of enlarging swept volume by the mobility control effect. Then microetching model was used to carry out experiments to observe the phenomenon of enlarging swept volume by surface-active polymer flooding. In addition, the pattern of plugging and enlarging swept volume by surface-active polymer emulsion was analyzed, and experiments on plugging effect of surface-active polymer were conducted to evaluate the performance of enlarging swept volume by surface-active polymer flooding.
3.1. Mobility control effect of surface-active polymer
In chemical flooding, the mobility control refers to controlling the mobility of the displacing phase close to or less than the mobility of the displaced phase, which is one of basic principles of chemical flooding to enhance oil recovery by improving the viscosity of the displacing phase.
3.1.1. Mechanism of enlarging swept volume by mobility control effect
Surface-active polymer increases the viscosity and flow resistance of displacing phase, so the mobility of displacing phase decreases. Meanwhile, surface-active polymer has little impact on the viscosity of oil, so the oil phase permeability increases, and the mobility of oil increases, so the mobility ratio of the displacing phase and displaced phase decreases and then swept volume enlarges[13].
Resistance coefficient can be used to evaluate the mobility control effect of chemical agents, and under the precondition of little change in oil and water viscosity in oil-bearing pores, the higher the resistance coefficient, the stronger the mobility control effect. The experimental results (Table 4) show that the resistance coefficient increases with the increase of surface-active polymer concentration, so surface-active polymer with higher concentration has stronger capacity to control mobility. The viscosity of surface-active polymer solution increases with the increase of concentration, the displacing phase drops in mobility and flow rate, resulting in obvious mobility control. The core with lower permeability has stronger mechanical capture effect to surface-active polymer, and thus the resistance coefficient increases.
Table 4 Experimental results of resistance coefficient of surface-active polymer solution.
| No. | Gas permeability of cores/10-3 μm2 | Concentration of surface-active polymer/(mg·L-1) | Resistance coefficient | |
|---|---|---|---|---|
| Measurement value | Average value | |||
| 1 | 100 | 750 | 168 | 119.3 |
| 2 | 200 | 120 | ||
| 3 | 500 | 70 | ||
| 4 | 100 | 1000 | 268 | 227.7 |
| 5 | 200 | 245 | ||
| 6 | 500 | 170 | ||
| 7 | 100 | 1250 | 415 | 303.7 |
| 8 | 200 | 279 | ||
| 9 | 500 | 217 | ||
| 10 | 100 | 1500 | 1181 | 917.7 |
| 11 | 200 | 898 | ||
| 12 | 500 | 675 | ||
| 13 | 100 | 2000 | 1899 | 1250 |
| 14 | 200 | 1113 | ||
| 15 | 500 | 738 | ||
Table 5 Experimental results of contribution rate of mobility control effect of surface-active polymer flooding for enhanced oil recovery.
| Water flooding recovery/% | Viscosity/ (mPa·s) | Polymer flooding | Surface-active polymer flooding | Contribution rate of mobility control effect/% | ||
|---|---|---|---|---|---|---|
| Recovery/% | Increase/% | Recovery/% | Increase/% | |||
| 23.67 | 45 | 48.4 | 24.7 | 56.2 | 32.5 | 76.02 |
| 100 | 48.8 | 25.1 | 56.5 | 32.8 | 76.55 | |
| 200 | 49.5 | 25.8 | 56.7 | 33.0 | 78.20 | |
| 300 | 50.4 | 26.7 | 57.1 | 33.4 | 79.96 | |
| 400 | 51.7 | 28.0 | 58.3 | 34.6 | 80.94 | |
3.1.2. Contribution rate of mobility control effect to recovery
Surface-active polymer has both viscosity-increasing property and surface activity, so mobility control effect brought about by viscosity increase can increase sweep efficiency, and surfactant can enhance oil-washing capacity and in turn oil washing efficiency, the two both have contribution to oil recovery enhancement in surface-active polymer flooding.
Organic chromium crosslinking polymer with viscosity same to the surface-active polymer was selected to conduct oil displacement experiments to test the effect of mobility control and oil-washing capability of surface-active polymer on enhancing oil recovery. The man-made core used in the experiment is made of quartz cemented by epoxy resin, and is 300 mm×45 mm×45 mm and 200×10-3 μm2 in gas permeability. The experiment was done at the temperature of 45 °C and the formation water salinity of 3700 mg/L. The oil used in the experiment was a mixture of dehydrated crude and kerosene with a viscosity of 9.8 mPa·s.
With similar viscosity, the two chemical agents have similar mobility control effect, then it is deemed that the oil recovery difference between them is caused by the difference in their oil-washing abilities. The ratio of oil recovery increase by chromium crosslinking polymer flooding to oil recovery increase by surface-active polymer flooding is defined as contribution rate of mobility control effect of surface-active polymer flooding to enhanced oil recovery.
The results show that the average oil recovery of waterflooding was 23.67%. Mobility control effects of chromium crosslinking polymer solution and surface-active polymer solution enhance with the increase of viscosity, the sweep efficiencies rise and oil recovery increase significantly. In surface-active polymer flooding, contribution rate of mobility control effect to enhanced oil recovery is more than 76%, so the contribution rate of mobility control effect to enhanced oil recovery is much greater than the contribution rate of oil-washing capacity.
3.2. Microscopic experiments on enlarging swept volume
The mobility control effect can make surface-active polymer enter oil-bearing pores that cannot be swept by water flooding to drive residual oil. Microscopic oil displacement experiments was carried out on a microetching model to observe the phenomenon of enlarging swept volume by surface-active polymer flooding. Microscopy was used to observe and record the oil displacement process during surface-active polymer flooding to compare qualitatively and analyze quantitatively the microscopic distribution rule of remaining oil and the sweeping status of surface-active polymer.
The pore network structure on the cast thin section of natural core was reproduced by optical engraving method, a microetching model was made through processes of plate making, glue coating, imaging, etching and sintering. The model was an injection-production unit which was one-fourth of a five-point well pattern, and the two holes at the diagonal were set as the injection and production wells. The model is 45 mm×45 mm on the plane, with pores of 0.1-100.0 µm.
Experimental procedure: The microetching model was saturated with formation water after vacuumized, and then saturated with simulated oil by micro-pump. Residual oil after water flooding were formed by water flooding at constant pressure and speed till water content was 100%. The residual oil was displaced by surface-active polymer solution, then by water flooding again until there was no oil in the produced liquid. The displacement process was recorded by microscopy and high speed camera to compare the oil and water distribution.
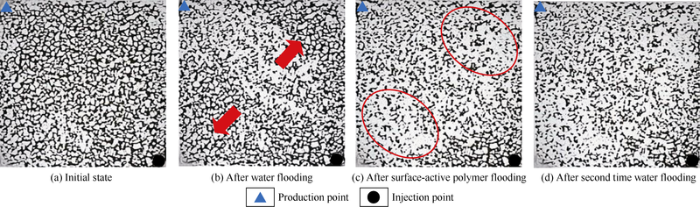
As shown in Fig. 20, a certain proportion of oil was driven out after the water flooding, but the non-mainstream line area in the model still had a large amount of residual oil, and in particular the corners of the model were full of residual oil (marked by red arrows in Fig. 20b). Residual oil in the corners of the model reduced significantly after surface-active polymer flooding (the red circle in Fig. 20c), which shows the surface-active polymer can enter oil-bearing pores not swept by waterflooding, to carry out the oil. Whereas the distribution of oil and water changed little after the second time of water flooding (Fig. 20d), suggesting little displacement effect.
Fig. 20.
The distribution of oil and water in different displacement stages.
The proportions of swept area of different displacement stages were calculated and compared by planar mesh partitioning of the microetching model (Table 6). The proportion of swept area after water flooding was 22.3%, the proportion after surface-active polymer flooding increased to 69.8%, that is an increase of 47.5% than water flooding. The proportion of swept area after second time water flooding was 72.3%, only 2.5% increase from the surface-active polymer flooding.
Table 6 The proportions of swept area after floodings by different displacement agents.
| Displacement mode | Proportion of swept area | Difference of the swept area proportion/% |
|---|---|---|
| Water flooding Surface-active polymer flooding Second time water flooding | 22.3 69.8 72.3 | 47.5 2.5 |
3.3. Macroscopic evaluation of enlarged swept volume by emulsion plugging
3.3.1. Model of enlarging swept volume by emulsion plugging
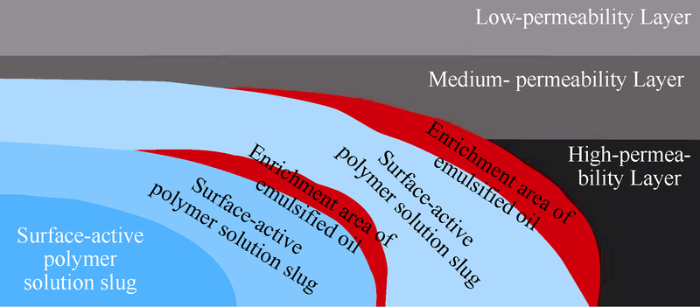
The surface-active polymer solution was low in viscosity initially, so it was easy to enter into the deep formation. As the solution flew into the formation, the viscosity of surface-active polymer solution gradually restored and increased, as the self-crosslinking characteristic of the surface-active polymer started to work. A slug with high viscosity was formed by surface-active polymer solution in the front edge of displacing phase (Fig. 21). Due to the strong emulsification effect of surface-active polymer, crude oil was emulsified to form emulsion with high viscosity. The emulsion was pushed, gathered and retained with the displacing phase to form a large volume enrichment area of emulsified oil, which further greatly increases the flow resistance of the front edge of displacing phase. Finally, a strip with plugging effect was formed by the mixing of the high viscosity slug of surface- active polymer solution and the enrichment zone of emulsified oil. The plugging strip has high viscosity but not high overall strength, so the plugging strip slowly moved to deeper part of the formation driven by water injected later. This dynamic process can enlarge the swept degree and macroscopic swept volume significantly.
Fig. 21.
Model of enlarging swept volume by emulsion plugging.
3.3.2. Experiments on emulsification plugging effect of surface-active polymer
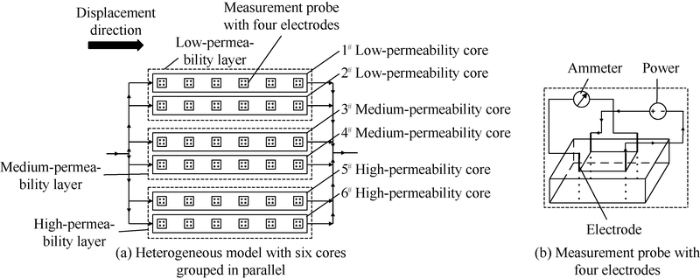
The vertical heterogeneity of reservoir was simulated by artificial cores with different permeabilities grouped in parallel, and a three-layer model of low-medium-high permeability artificial cores grouped in parallel was established (Fig. 22). In order to find out the difference in utilization within one layer, each layer was made up of two artificial cores in parallel. The cores used in the experiment were made by quartz cemented by epoxy resin of 300 mm×45 mm×45 mm, with a gas permeability of 100×10-3 μm2, 200×10-3 μm2 and 1000×10-3 μm2.
Fig. 22.
Heterogeneous models with six cores grouped in parallel.
Six measurement probes were set on each core to test oil and water saturation, and each probe had four electrodes. Experiments were conducted at the temperature of 45 °C and formation water salinity of 3700 mg/L. The experimental oil was a proportional mixture of degassed crude oil and kerosene, with a viscosity of 9.8 mPa s. Each core was vacuumized and saturated with water, then formation water was displaced with simulated oil till the core was saturated with oil. The displacement was at a constant injection rate of 1.2 mL/min. After the core was water flooded to water content of 100%, a surface-active polymer slug of 1.0 PV (injection pore volume) was injected, finally, the model was water-flooded till no oil came out.
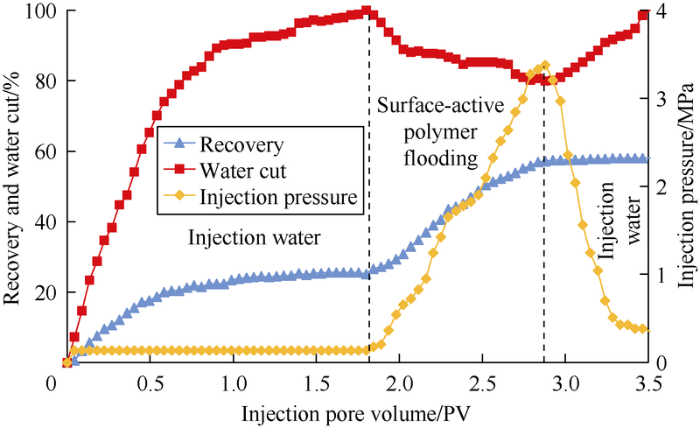
The experimental results show injection pressure increased suddently shortly after the beginning of surface-active polymer flooding after water flooding, which indicates that the viscosity-increasing property of surface-active polymer make the resistance of the displacing phase increase significantly (Fig. 23). The injection pressure rose dramatically constantly after a short period of steady slow increase, which indicates that the effect of emulsification plugging appears. The oil recovery increased and the water cut decreased in period of surface-active polymer flooding, suggesting the dominant channel in layer with high permeability was plugged and layers with low permeability were started to be produced. When the total injection volume reached about 2.88 PV, the surface-active polymer slug broke out in the outlet, and then the pressure dropped and water content rose.
Fig. 23.
Injection and production dynamic in displacement experiments on heterogeneous model with cores grouped in parallel.
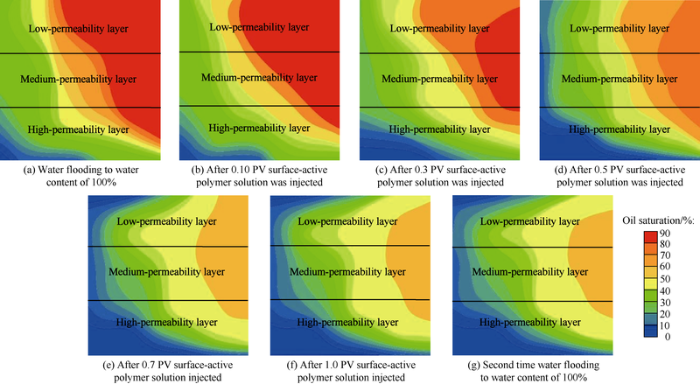
Oil and water saturation were measured by the preset probes with four electrodes, and then the vertical distribution of oil saturation in different stages of flooding were plotted (Fig. 24). (1) As the injected water increased, the high saturation front gradually advanced to the outlet end (Fig. 24a), the high permeability layer had better washing condition and lower average oil saturation, while the medium-low permeability layers had higher oil saturation. The oil saturations of medium and low permeability layers around the outlet end hardly changed, indicating that this place was not swept by injection water. (2) Oil saturation around the outlet end of the medium permeability layer began to change after 0.1 PV of surface-active polymer was injected, the oil saturation dropped somewhat (Fig. 24b), the medium permeability layer began to be produced under the effect of mobility control, but oil saturation changed little in other parts. (3) Injection pressure began to rise dramatically while the overall oil saturation of the whole model decreased after 0.3 PV of surface-active polymer was injected (Fig. 24c). Oil saturation of the production end of medium permeability layer decreased further, but was still in the high oil saturation range. (4) Injection pressure raised steadily and slowly after 0.5 PV of surface-active polymer solution was injected. The oil saturation of medium and low permeability layers decreased, and the overall oil saturation of the whole model decreased as well (Fig. 24d), and the oil washing efficiency increasing effect of surface-active polymer started to show. (5) After 0.7 PV surface-active polymer solution was injected, the position of high oil saturation part in the medium and high permeability layers changed little (Fig. 24e) and the oil saturation remained stable. Water saturation of low permeability layer began to increase, and injection pressure rose greatly at the same time. The middle part of medium and high permeability layers were plugged, whereas the low permeability layer began to be swept, showing remarkable emulsification and plugging effect of surface-active polymer. (6) After 1.0 PV surface-active polymer solution was injected, oil saturation in the lower permeability layer dropped significantly, high water saturation area in low permeability layer continued to advance toward the production end, and the overall oil saturation of the whole model further decreased in small amplitude (Fig. 24f). (7) After water content reached 100% in the second time water flooding, the oil and water distribution of the whole model hardly changed anymore (Fig. 24g).
Fig. 24.
Distribution of oil saturation in different stages of displacement.
4. Conclusions
Surface-active polymer is a novel chemical agent with both viscosity-increasing ability and surface activity suitable for high water-cut mature oilfields. Test results of the new type of surface-active polymer modified for Daqing placanticline oilfield show: the surface-active polymer differs obviously from ordinary polymer and polymer-surfactant binary system in molecular aggregation, performance of viscosity and flow capacity which has larger molecular coils, higher viscosity and viscosity-increasing property, and poorer transmission and flow capacity.
The surface-active polymer solution has good viscosity increasing and remaining performance, viscoelasticity and deformability, of which, viscosity increasing property and viscoelasticity can be made use to enhance oil recovery. The surface-active polymer can improve interface chemical properties, reduce oil-water interfacial tension, and make the reservoir rock turn water-wet. Moreover, the surface-active polymer can emulsify oil to form relatively stable oil-in-water emulsion. Under non-ultralow interfacial tension, emulsification capability is an important mechanism to enhance oil- washing efficiency.
The mechanisms of enlarging swept volume and enhancing oil recovery by surface-active polymer flooding have two aspects. Microscopically, surface-active polymer has mobility control effect, and can enter oil-bearing pores not swept by water to displace out residual oil, and its mobility control effect has much more contribution to enhance oil recovery than its oil washing capacity. Macroscopically, emulsification and plugging capability of surface-active polymer make contribution to pulgging the layer with high permeability, forcing the injected fluid later to divert to the medium or low permeability layers with lower flow resistance, thus enlarging swept volume.
Nomenclature
Dh—molecular coil size, nm;
l, m, n—polymerization degree of surface-active polymer molecule, dimensionless;
p1—pressure at the first trisection, MPa;
p2—pressure at the second trisection, MPa;
pc1—capillary force before surface-active polymer flooding, MPa;
pc2—capillary force after surface-active polymer flooding, MPa;
pin—pressure at the injection end, MPa;
pout—pressure at the production end, MPa;
Δp—displacement pressure difference, MPa;
θ1—wetting angle before surface-active polymer flooding, (°);
θ2—wetting angle after surface-active polymer flooding, (°).
Reference
Current situation and development strategy of the extra high water cut stage of continental facies sandstone oil fields in China
New progress and prospect of oilfields development technologies in China
Combined effects of polymer/surfactant/oil/alkali on physical chemical properties
DOI:10.1016/j.desal.2005.05.013 URL [Cited within: 1]
A novel surface active polymer oil displacement agent
Based on the relationship between the molecule structure of monomers and their performance, two types of polymerizable monomers with low oil/water interfacial tension have been synthesized, which coplymerizes acrylamide to synthesize a novel polymer oil displacement agent under compound initiator and low polymeric temperature. Its structure and distribution status in brine solution were characterized by infrared spectroscopy and freeze etching electron microscope. The results show that the polymer has good water solubility, viscosifying properties, salt-resistance and temperature-tolerance, shearing-resistance in brines. Due to the graft polymerization of acrylamide and the polymerizable surface-active monomers, the polymer overcomes chromatographic separation effect, and has low oil/water interfacial tension. Interfacial tension of 0.15% polymer solution (prepared in Daqing simulation brine) is around 10(-1) mN/m in Daqing No. 1 oil plant crude oil. Core flooding experiments show that the oil recovery of the novel surface active polymer is 5.2% higher than that of ordinary hydrolyzed polyacrylamide because of its better viscosifying performance and lower oil/water interfacial tension.
The variation laws of seepage of hydrophobic associating polymer flooding after polymer flooding in onshore oilfields
Differences in molecular configuration and seepage properties among polymer, active polymer and Cr 3+ polymer gel
Study on the mechanism of polymer solution with viscoelastic behavior increasing microscopic oil displacement efficiency
The role of viscoelasticity of alkali/surfactant/polymer solutions in enhanced oil recovery
DOI:10.1016/j.petrol.2005.04.001 URL [Cited within: 1]
Effect of elasticity during viscoelastic polymer flooding: A possible mechanism of increasing the sweep efficiency. SPE 133471
Study of polymer-surfactant interactions via surface tension measurements
DOI:10.1007/s003960000455
URL
[Cited within: 1]

Interactions between a polymer and a surfactant were studied via surface tension measurements. Poly(ethylene glycol) and sodium dodecyl sulfate were used as a polymer and a surfactant, respectively. The addition of polymer affected the CMC value of the surfactant. The interpretations of the data and theoretical plots of polymer-surfactant interactions are discussed using a theoretical model.
Stability mechanism of W/O crude oil emulsion stabilized by polymer and surfactant
DOI:10.1016/j.colsurfa.2011.05.017 URL [Cited within: 1]
Wettability altering secondary oil recovery in carbonate rocks
DOI:10.1016/j.saa.2019.117936
URL
PMID:31864151
[Cited within: 1]

TiO2 nanoparticles as solar cells and photocatalysts caused extensive attention in solar energy utilization and environment remediation due to the high photoelectrochemical performance. We demonstrated a novel approach to fabricate big-leaf hydrangea-like Bi2S3-BiOBr self-assembled by superthin nanosheets on TiO2 nanotube arrays (TiO2 NTs/B2S3-BiOBr). Results indicated that the Bi2S3-BiOBr co-sensitization showed higher photoelectric conversion efficiency than the single Bi2S3 or BiOBr sensitization. More remarkably, TiO2 NTs/B2S3-BiOBr showed excellent photoelectrocatalytic (PEC) removal of MB, MO, RhB and Cr(VI). The remarkable PEC performance could be attributed to the strong visible light absorption and effective electron transportation at the interface of TiO2/B2S3-BiOBr. The high photoelectrochemical performances indicate that the TiO2 NTs/B2S3-BiOBr could work as potential photoelectric materials for large-scale applications in the photoelectrochemical energy conversion and pollutant removal.
Capillary-driven mobility control in macro emulsion flow in porous media
DOI:10.1016/j.ijmultiphaseflow.2012.03.001
URL
[Cited within: 1]

We show that the mobility of an emulsion with drops larger than the porous throats is a strong function of the local capillary number; it falls as the interfacial forces become stronger (low capillary number). Emulsion drops have little effect on the fluid mobility at high capillary number. This flow behavior can be used as a selective mobility control mechanism driven by capillary forces. The flow rate and emulsion characteristics can be selected in such a way that fluid mobility near the injecting source remains high. allowing the emulsion to reach the location where the mobility control is needed. To prove the benefit of capillary-driven mobility control, we study the displacement of mineral oil by water and emulsion at two capillary numbers by measuring the volume of displaced fluid. Higher efficiency displacement due to high mobility of the displacing fluid only occurs at low capillary number. (C) 2012 Elsevier Ltd.