Introduction
Great success has been made in recovery of shale oil in the US, which tremendously boosts exploration and exploitation of shale oil all over the world[1,2,3,4,5,6,7,8]. Substantial achievements have been made in the development of marine shale gas in China, meanwhile development of continental shale oil has drawn extensive attention in this industry[9,10,11]. In general, there are two types of shale oil of continental shale in China owing to its thermal evolution degrees, namely the mid-high maturity and mid-low maturity ones. In recent years, extensive studies have been carried out for mid-high maturity shale series, specifically with respect to the hydrocarbon resource potential, retained hydrocarbon content, and shale pore structure, etc.[12,13,14]. With application of horizontal well network fracturing technology, important discoveries have been achieved in several basins, demonstrating good exploration prospect of shale oil of mid-high maturity. Due to lower maturity and limited hydrocarbons generated, the organic-rich shale series with mid-low maturity is abundant in unconverted organic matters, so they have huge potential of generating hydrocarbon through artificial heating. Since 2005, CNPC and Shell have started their collaboration in research in this field. They have evaluated the in-situ conversion potential of organic-rich shale with mid-low maturity, and worked out some in-situ thermal conversion methods. The research shows that organic-rich shale suitable for in-situ conversion should have a total organic carbon (TOC) of over 6%, and vitrinite reflectance (Ro) of 0.5-1.0%[10]. The technically recoverable resources of the mid-low maturity continental shale oil and shale gas in China by in-situ conversion technology are about (700-900)×108 t and (60-65)×1012 m3 respectively, representing significant potential.
In-situ heating consumes other energy sources. Generally speaking, the higher the heated temperature and the longer the heating duration, the more energy will be consumed. It should be noted that thermal conductivity of rock isn’t discussed in this paper, and thus the energy required by in-situ heating cannot be accurately calculated. During underground in-situ heating of shale with mid-low maturity, if the heated temperature is too low or heating duration too short, organic matter can’t generate hydrocarbon massively. On the contrary, too high temperatures or too long heating duration may result in over-heating, which not only increases unnecessary energy consumption but also causes massive liquid hydrocarbons cracking into gas and reducing of economic benefit. Given these, identification of the optimal heating temperature or the heating procedure so as to achieve the highest hydrocarbon conversion ratio with the least energy consumption is the key to improving the economic benefit of development of mid-low maturity shale.
The hydrocarbon generation kinetics is an important constituent of the oil and gas generation theory. Its core principle is the time-temperature complementary effect represented by the Arrhenius equation, in which the hydrocarbon generation process of the source rock is regarded as several in-series or parallel chemical reactions, and parameters of the hydrocarbon generation kinetics of the source rock are obtained through the laboratory simulation facilitated by rapid heating rate and used to calculate the hydrocarbon generation conversion ratio in the case of the slow heating rate under the geological condition. Findings of such research can provide important references for investigating the hydrocarbon generation temperature and time[15,16,17,18,19,20,21,22,23,24,25]. The underground in-situ heating conversion of shale oil is similar to hydrocarbon generation kinetic simulation in laboratory. In this study, the theory and methodology of hydrocarbon generation kinetics were used to compute the in-situ conversion ratio of shale. The hydrocarbon generation kinetic parameters were acquired via the laboratory simulation, and then the temperature and time required for hydrocarbon generation of organic-rich shale with mid-low maturity by in-situ artificial heating were calculated to provide references for shale in-situ conversion.
1. Samples and experiments
In accordance of the appraisal of the key areas in China that are applicable to in-situ conversion, this paper collects four organic-rich shale core samples from the Nen-1 Member of the Cretaceous Nenjiang Formation in the northwestern Songliao Basin (Sample No. 1), the Chang-7 Member of the Trassic Yanchang Formation in the southwestern Ordos Basin (Sample No. 2), the upper member of the Paleogene Lower Ganchaigou Formation in the Qaidam Basin (Sample No. 3) and the Permian Lucaogou Formation of the Jimusar Sag in the eastern Junggar Basin (Sample No. 4) with shallow burial depths, low maturity and high oil generation potential were selected for experiments. The information of the samples is listed in Table 1.
Table 1 Intial geochemical parameters of the source rock samples.
| Sample No. | Sample Type | Basin | Formation | Depth/ m | TOC/ % | Tmax/ °C | Ro/ % | S1/ (mg•g-1) | S2/ (mg•g-1) | S3/ (mg•g-1) | (S1+S2)/ (mg•g-1) | S2/S3 | (S2/TOC)/ (mg•g-1) | (S3/TOC)/ (mg•g-1) | (S1/TOC)/ (mg•g-1) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Shale | Songliao | Nen-1 | 500 | 7.76 | 430 | 0.46 | 10.70 | 59.12 | 1.21 | 69.82 | 49 | 795 | 16 | 138 |
| 2 | Shale | Ordos | Chang-7 | 370 | 19.17 | 430 | 0.52 | 4.01 | 96.19 | 0.53 | 100.2 | 181 | 502 | 3 | 21 |
| 3 | Shale | Qaidam | Upper Member of Lower- | 3345 | 4.69 | 436 | 0.54 | 9.58 | 32.79 | 0.42 | 42.37 | 78 | 699 | 9 | 204 |
| 3 | Kerogen | Ganchaigou | 34.84 | 438 | 0.56 | 3.13 | 292.78 | 0.87 | 295.91 | 337 | 840 | 2 | 9 | ||
| 4 | Shale | Jungar | Lucaogou | 3126 | 6.37 | 440 | 0.68 | 1.06 | 53.50 | 4.56 | 54.56 | 12 | 840 | 11 | 17 |
First, conventional rock pyrolysis, TOC and Ro tests were conducted on the four samples to acquire their basic geochemical parameters related to the organic matter abundance, type and maturity[25]. Subsequently, the kinetic simulation of hydrocarbon generation was conducted, using the Rock-Eval 6 analyzer. The heating procedure is as follows: The analyzer temperature was rapidly elevated to 300 °C and kept constant for 3 min to remove free hydrocarbons. Then, the temperature was heated to 600 °C at the rate of 10 °C/min, 20 °C/min, 30 °C/min, 40 °C/min and 50 °C/min respectively, to acquire the hydrocarbon production efficiency curves of the samples at different heating rates, which are basic data for calculation of the hydrocarbon generation kinetics. The geological body is a semi-open system. During the underground in-situ heating conversion of the source rock, the generated oil and gas, on the one hand, are expelled out from the source rock into the surrounding interbeds of siltstone or sandstone, especially in the case of high temperatures and pressures seen with higher hydrocarbon expulsion efficiencies. On the other hand, there are also present active development wells recovering the generated oil and gas in a timely manner. Consequently, during the in-situ conversion of shale, the hydrocarbon expulsion channel should be open. Oil and gas are mainly originated from primary cracking of kerogen, while the oil and gas from secondary cracking of liquid hydrocarbons take a limited share. Given this, the kinetic parameters from simulation with the Rock-Eval analyzer can be used for in-situ heating of shale.
To compare the hydrocarbon production efficiency of source rock, the sample from the western Qaidam Basin (No. 3) was treated and made into kerogen (using hydrochloric acid and hydrofluoric acid to remove most of minerals). Gold-tube thermal simulation experiment was conducted on this sample, the heating process is as followings: the inner temperature of the gold tube was rapidly raised up from the room temperature to 250 °C and kept constant for 30 min. Furthermore, it is increased to the target temperatures at a rate of 20 °C/h, after which the tube was quickly cooled down to the room temperature. The set target temperatures were 300 °C, 310 °C, 320 °C, 330 °C, 340 °C, 350 °C, 360 °C, 370 °C, 380 °C, 390°C, 400 °C, 410 °C, 420 °C, 430 °C, 440 °C, 450 °C, 475°C and 500 °C. After cooled down to the room temperature, the gold tube was cut open, and liquid hydrocarbons were extracted with dichloromethane, to obtain the quantity of oil generated from kerogen under different temperatures.
The aforementioned experiments were all accomplished in the CNPC Key Laboratory of Petroleum Geochemistry.
2. Experimental results
The results of rock pyrolysis and TOC measurement are presented in Table 1. It can be seen the four samples all have high organic matter abundance. Sample 3 has a TOC of 4.69% and kerogen TOC of 34.84%. The other three samples all have TOC of more than 6%, up to 19.17%, which meet the organic matter abundance criterion for in-situ heating conversion. The rock samples all show high contents of pyrolysis hydrocarbons (S2), of over 30 mg/g, up to 96.19 mg/g. This indicates considerably substantial hydrocarbons generated by these source rocks after high-temperature heating. The hydrocarbon generated per unit TOC (IH) is also high. The Sample 2 with Type-II1 organic matter, is the lowest in this value (502 mg/g); while the other samples are all over 600 mg/g in IH, with the highest of 840 mg/g, representing Type-I organic matter. All the samples have type indexes (S2/S3) of more than 5, indicating they all have oil-prone organic matter. All the samples have low residual free hydrocarbon contents (S1), mostly below 10 mg/g, and S1/TOC below 50 mg/g, suggesting they have limited quantity of free hydrocarbons when un-heated. Sample 3 has higher S1/TOC, which may be related to the early hydrocarbon generation of the source rock of salinized lake facies.
The maturity of source rock is an important index in the hydrocarbon generation potential evaluation of organic-rich shale. Vitrinite reflectance of the four samples were all measured, but as the lacustrine source rock has lower content of vitrinite, and the testing points of each sample was less than 10, the results can only be used for reference. The maturity of the four samples was judged preliminarily based on the rock pyrolysis data. Samples 1, 2 and 3 all have Tmax around 435 °C, very high IH and very low S1, which suggest that they are at the immature stage. Sample 4 has a Tmax of 440 °C, IH up to 840 mg/g and S1/TOC of only 17 mg/g, indicating that it is in low maturity stage.
On the basis of the hydrocarbon generation simulation, the relationship between the hydrocarbon conversion ratio and heating temperature and duration under the heated geological conditions can be simulated by using hydrocarbon-generation kinetic approach. In this study, the parallel first-order reaction was adopted. Its basic assumption is that the hydrocarbon generation process of kerogen can be divided into hydrocarbon generation from several different components (corresponding to different kerogen types). These components have independent parallel chemical reactions, and each reaction possesses its unique thermal stability, namely unique hydrocarbon generation kinetic parameters. Hydrocarbon generation of kerogen is the summation of hydrocarbon generation reactions of all the components, and the contribution of reaction of each component to the overall hydrocarbon generation reaction is determined by the abundance of the specific component. The reaction can be expressed by the following equation[15,16]:
Based on hydrocarbon production efficiency curves obtained at different heating rates, the parameters of the source rock such as the activation energy and pre-exponential factor were calculated using the kinetic simulation software. For the detailed computation process of the hydrocarbon generation kinetics of the software, please refer to references [15-18].
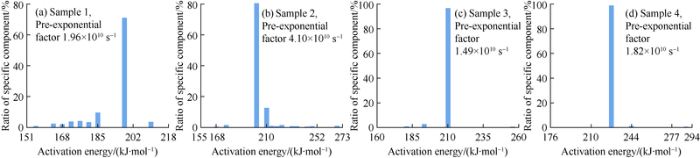
Through the fitting calculation with the hydrocarbon generation kinetics software, the activation energy and pre-exponential factor of the four samples were obtained (Fig. 1). Sample 1 has the lowest and relatively scattered pyrolysis activation energy values from 155 kJ/mol to 210 kJ/mol, with the main peak of 197 kJ/mol and weighted average of 192.61 kJ/mol, and pre-exponential factor of 1.96×1010 s-1. Sample 2 is similar to Sample 3 in activation energy. Its main peak of activation energy is 202 kJ/mol, accounting for 80% of the weight, while its secondary peak is at 210 kJ/mol, accounting for 12%. Its weighted average of activation energy is 204 kJ/mol, and its pre-exponential factor is 4.10×1010 s-1. Sample 3 has the main peak of activation energy of 210 kJ/mol, accounting for 96%, weighted average activation energy of 209.45 kJ/mol, and pre-exponential factor of 1.49×1011 s-1. Sample 4 has the highest and most concentrated activation energy, with the main peak at 227 kJ/mol, accounting for 99%, weighted average of 227.09 kJ/mol, and the pre-exponential factor of 1.82×1012 s-1.
Fig. 1.
Histograms of activiation energy of the four samples.
Based on the above hydrocarbon generation kinetic parameters, the conversion ratio of in-situ hydrocarbon generation of the samples under other heating conditions can be calculated using the parallel first-order reaction model, Equations 3 and 4:
In this study, as heating was started from a fixed temperature, that is Tu= T0, calculation of Equation 3 cannot be accomplished. Given this, let T0 = Tu-1, and the calculation of Equation 3 can be done (for details, please refer to reference [19]).
Fig. 2 shows the measured and calculated conversion ratios of Sample 2 at five different heating rates. It can be seen that the measured and calculated values of the source rock conversion ratios at different heating rates are in good agreement, proving the results of calculation by the proposed method are basically reliable. Similar plots of the other three samples can also be obtained, and we do not need to repeat here.
Fig. 2.
Relationship between conversion ratio and heating temperature of Sample 2.
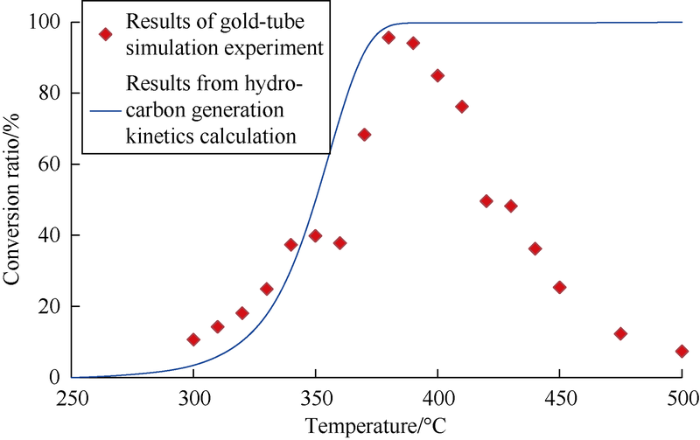
Comparing the simulation results of hydrocarbon generation kinetics with the simulation results of gold tube, it is found that results of the two methods are basically the same before 380 °C. When the temperature exceeds 380 °C, the liquid hydrocarbon would be cracked (Fig. 3).
Fig. 3.
Comparison of results from the gold-tube simulation experiment and hydrocarbon-generation kinetics calculation.
Taking Sample 3 as an example, the gold-tube simulation experiment shows that the sample starts to produce a small amount of liquid hydrocarbon at 300 °C, then the amount of liquid hydrocarbon produced gradually increases, and reaches the highest over 800 mg/g at 380 °C, with the hydrocarbon generation conversion ratio of nearly 96%. Subsequently, liquid hydrocarbon begins cracking, and the total oil produced tends to decline, and drops below 200 mg/g at 450 °C. According to the hydrocarbon generation kinetics computation, the hydrocarbon generation conversion ratio of the sample is lower than 10% at the temperature below 300 °C, and reaches 50% at 350 °C and 98% at 380 °C.
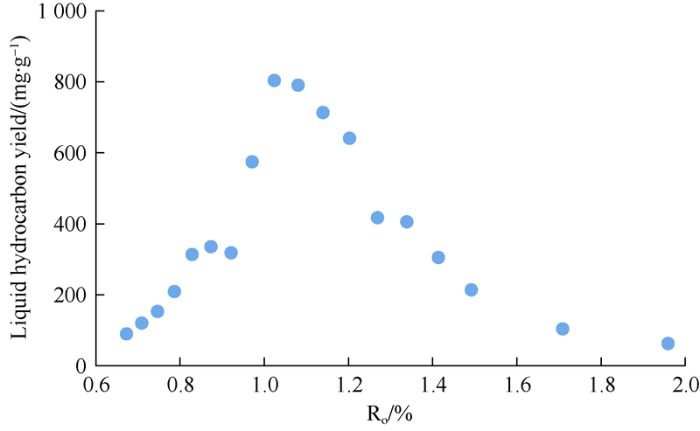
Through vitrinite reflectance conversion, Ro of the sample at its main oil generation stage is of 0.7-1.0%, and liquid hydrocarbons start to crack after Ro surpasses 1.0%. When Ro exceeds 1.5%, the production of liquid hydrocarbons drops below 200 mg/g (Fig. 4). This result is consistent with the classical oil and gas generation model, which validates the credibility of the hydrocarbon generation simulation and gold-tube simulation results.
Fig. 4.
Relationship between liquid hydrocarbon yield and Ro of Sample 3.
3. Discussion
3.1. Relationship between activation energy of hydrocarbon generation and maturity
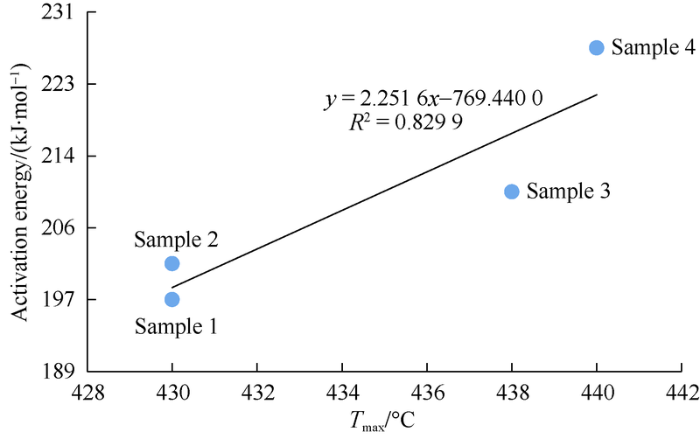
Both the activation energy of hydrocarbon generation by source rock cracking and the peak temperature (Tmax) of rock pyrolysis reflect the difficulty of cracking and hydrocarbon generation of organic matters. They have a good linear correlation, with a correlation coefficient close to 0.83 (Fig. 5). Owing to the limited number of measurement points, the universality of the linear correlation needs to be validated by analysis of more samples.
Fig. 5.
Relationship ow activation energy with peak temperature of the four samples.
Maturity also affects the concentration degree of activation energy distribution. From the four typical samples, Sample 1, with the lowest maturity, has the lowest activation energy and most disperse distribution frequency. Its main peak weight is 70.84%, while its weight for activation energy lower than the main peak equals to 25.67%. Sample 4, with the highest maturity, has the highest activation energy, and the main peak weight of 98.86%. This may imply that during the early hydrocarbon generation stage of immature organic matter, the soluble organic matter makes some contribute, and when entering low mature-mature stage, mainly kerogen generates hydrocarbon, with correspondingly concentrated activation energy.
3.2. Effect of heating rate on organic matter conversion rate
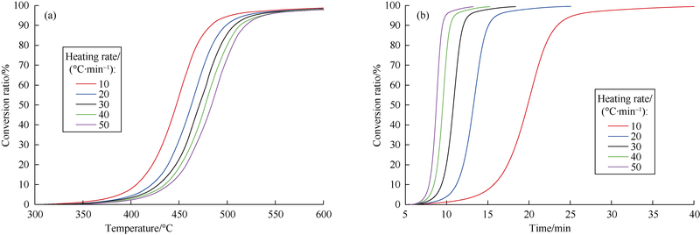
Sample 2 is taken as the example to illustrate the correlation between the organic matter conversion ratio, heating temperature and duration obtained via Rock-Eval-based thermal simulation (Fig. 6). It can be seen the five curves of heating temperature with conversion ratio are basically consistent in trend. The hydrocarbon generation sections are generally parallel with each other, with the line section in the case of a slower heating rate turning up on the left and that corresponding to a faster heating on the right. The lower the heating rate, the lower the temperature will be needed to reach the same conversion ratio. For instance, to reach the conversion ratio of 50%, the temperature needs to reach 450 °C in the case of the heating rate of 10 °C/min, while the temperature needs to reach 480 °C at the heating rate of 50 °C/min, with a disparity of 30°C. In contrast, the five curves of heating time with conversion ratio have considerable differences. The faster the heating rate, the steeper the curve; the curve with fast heating rate appears on the left, and the curve with slow heating rate appears on the right. The slower the heating rate, the longer the time is needed to reach the same conversion ratio. For example, to reach the conversion ratio of 50%, it takes only 8 min at a heating rate of 50 °C/min, and it takes 20 min at the heating rate of 10 °C/min.
Fig. 6.
Conversion ratio of hydrocarbon genration vs. heating temperature (a) and heating time (b) of Sample 2, at different heating rates.
3.3. Temperatures for underground constant-temperature heating conversion of shale
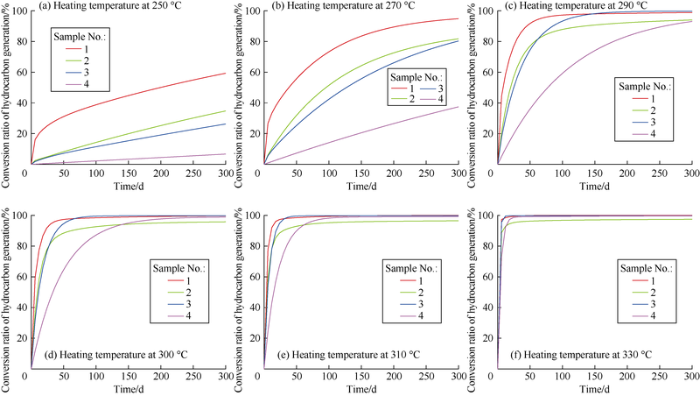
There are two heating methods: one is constant-temperature, and the other is constant-rate heating. For constant-temperature heating, determination of the heating temperature is of utmost importance. On the basis of the hydrocarbon generation kinetic parameters of the four samples calculated previously, the conversion ratios of the four samples at the heating temperatures of 250 °C, 270 °C, 290 °C, 300 °C, 310 °C and 330 °C were computed respectively. In a relatively prolonged heating duration (the maximum assumed time in this study for calculation and simulation was 300 d), the conversion ratio of the four samples with time were plotted (Fig. 7).
Fig. 7.
Relationship of conversion ratio of hydrocarbon generation and heating time of the four samples.
It can’t effectively facilitate hydrocarbon conversion with too low heating temperature. For example, after heated at the temperature of 250 °C for 300 d, all samples have conversion ratios lower than 40%, except for Sample 1 (60%) (Fig. 7a). When heated at the temperature of 270 °C, Sample 1 reaches a conversion ratio of 95% after heated for 300 d. Whereas, Samples 2 and 3 have conversion ratios of about 80%, and Sample 4 has a conversion ratio of still below 40% (Fig. 7b). When the heating temperature rises to 300 °C, Samples 1, 2 and 3 all reach conversion ratios of over 90% only heated for 50 d, and Sample 4 reaches conversion ratio of over 65% (Fig. 7d). When the heating temperature is 330 °C, Sample 1, 2 and 3 reach conversion ratios of over 90% within just 5 d, and Sample 4 reaches the conversion ratio of 90% after 10 d of heating (Fig. 7f).
The conversion ratio of hydrocarbon generation is positively correlated with the heating time, in other words, the longer the heating time, the higher the conversion ratio. However, it is not a simple linear correlation. Instead, as heating proceeds, the conversion ratio first rapidly grows in the early stage, and then grows slowly in the late stage. Moreover, the four samples differ widely in conversion ratio. Under the laboratory conditions, 300 °C is regarded as the temperature for volatilization of free hydrocarbons at which kerogen wouldn’t crack to generate additional hydrocarbons in a short time. Through our calculation based on the kinetics, if the heating time is long enough, conversion ratio of hydrocarbon generation of kerogen will be fairly high at 300 °C. After 5 d of heating, the Sample 1 reached a conversion ratio of 50%, while Samples 2 and 3 reached a conversion ratio of 25%, and Sample 4 reached a conversion ratio of 20%. After 30 days of continuous heating, Sample 1, Sample 2, Sample 3 and Sample 4 reached conversion ratio of 94%, 82%, 83% and 67% respectively (Fig. 7d). Given these, in the case of constant-temperature heating, shale formations in different areas should be heated at different temperatures. For the samples in this study, the temperatures for continuous heating should be between 270 °C and 300 °C. At this temperature range, the conversion ratio can exceed 90% after 50-300 d of heating. If the temperature is too low (less than 270 °C), the conversion rate will be too low. If the temperature is too high (above 300°C), the shale can reach high conversion ratio in a short time, but this may result in over-heating and cracking of liquid hydrocarbons, and thus reduction of economic benefit.
3.4. Conversion temperatures of constant-rate heating of shale
In the case of heating at continuously rising temperature, the heating rate is an important parameter that needs to be determined for the in-situ conversion. In the pilot test of in-situ conversion of the Green river shale in the US carried out by Shell, the heating rate was 15 °C/month, and the temperature reached 350 °C after about two years of heating[26,27,28]. The hydrocarbon generation kinetics calculation suggests that lower heating rate (e.g. 3 °C/month) can lower down the temperature required for hydrocarbon generation (300 °C is enough to facilitate hydrocarbon generation), while at faster heating rate, higher temperature is needed to facilitate hydrocarbon generation (about 350 °C).
Sample 2 collected from the Ordos Basin was taken as an example to calculate the conversion ratios of hydrocarbon generation at the heating rates of 1 °C/month, 3 °C/month, 15 °C/month, 60 °C/month, 90 °C/month and 150 °C/month, respectively (Fig. 8). The initial temperature was 40 °C and the ending temperature was 400 °C. It can be seen that at lower heating rates (1 °C/month, 3 °C/month, 15 °C/month), the major hydrocarbon generation stage lies between 225 °C and 325 °C. At 225 °C, the conversion ratios are all below 5%, and all conversion ratios exceed 95% as the temperature reaches 325 °C (Figs. 8a-8c). In contrast, at higher heating rates (60 °C/month, 90 °C/month and 150 °C/month), the primary hydrocarbon generation stage is between 275 °C and 350 °C. The conversion ratios are lower than 10% at 275 °C, while all conversion ratios surpass 95% at 350 °C (Figs. 8d-8f).
In general, at constant heating-rate heating, the main hydrocarbon generation stage is between 225-350 °C. The faster the heating rate, the higher the corresponding temperature of the main hydrocarbon generation stage will be. Therefore, relatively high heating rate (e.g. 60-150 °C/month) should be selected so as to realize a higher hydrocarbon generation conversion ratio in a relatively short duration (2.5-6.0 months) and thus higher economic benefit.
Fig. 8.
Relationship of conversion ratio and heating temperature of Sample 2 at different heating rates.
The research was conducted on shale samples collected from four typical areas. However, since the samples collected from different sedimentary basins differ widely in sedimentary environments, types and enrichment degree of organic matter, and thermal evolution degree, they have big differences in the distribution of activation energy. This paper doesn’t cover the effects of sedimentary environment and characteristics of the hydrocarbon-generation component on the conversion ratio of hydrocarbon generation, and the results of simulation experiment and computation reflect the hydrocarbon generation characteristics of the tested samples. Therefore, prior to pilot test of shale in-situ conversion in an area, specific hydrocarbon generation kinetics simulation and calculation are recommended to obtain more reasonable kinetic parameters to provide references for making a more appropriate heating plan for the in-situ heating conversion.
4. Conclusions
The continental shale formations in different areas have some differences in hydrocarbon generation kinetics parameter. The study on the samples in this work shows maturity is an important decisive factor of in-situ conversion of shale; the higher the maturity, the higher and more concentrated the activation energy of the hydrocarbon generation reaction will be.
The hydrocarbon generation kinetics simulation can provide important parameters for calculating the shale with mid-low maturity conversion ratio of in-situ heating. In the case of constant-temperature heating, the ideal heating temperature ranges between 270 °C and 300 °C. Temperatures too low leads to excessively slow conversion rate of hydrocarbon generation. Temperatures too high may result in over-heating and unnecessary energy consumption, although the conversion ratio can reach 90% within a short time.
For the heating at continuously rising temperature, the heating rate is an important parameter needed to be worked out. In general, in the case of the heating rate range of 1-150 °C/month, the temperature corresponding to the main hydrocarbon generation stage is from 225 °C to 350 °C. In order to improve the economic benefit, higher heating rates (e.g. 60-150 °C/month) are preferred, which does not only avoid waste of energy during heating but also realizes higher conversion ratio of hydrocarbon generation within a relatively short term (2.5-6.0 months).
This paper incorporates no discussion of the effects of sedimentary environment and characteristics of hydrocarbon-generation components on the conversion ratio of hydrocarbon generation, and the obtained results of simulation experiment and computation only reflect the hydrocarbon generation characteristics of the tested samples. Therefore, before implementation of pilot test of shale in-situ conversion in an area, specific hydrocarbon generation kinetics simulation and calculation are suggested, so as to yield more reasonable kinetic parameters, and subsequently a more appropriate heating plan for the in-situ heating conversion.
Nomenclature
Ai—frequency factor of the hydrocarbon reaction of the i-th component, s-1;
D—heating rate, °C/min;
Ei—activation energy of the reaction of the i-th component, kJ/mol;
ki—reaction rate of the i-th component, mol/min;
R—gas constant, 8.314 J/(mol•K);
t—reaction time, min;
T—heating temperature, °C;
T0—initial temperature, °C;
Tu—ending temperature, °C;
X—total conversion ratio of kerogen, %;
Xi—conversation ratio of the i-th component,%
Xoi—original abundance of the i-th component, %.
Reference
Statistical review of world energy 2018
Mississippian Barnett Shale: Lithofacies and depositional setting of a deep-water shale-gas succession in the Fort Worth Basin, Texas
Shale resource systems for oil and gas: Part 2: Shale-oil resource systems: BREYER J A. Shale reservoirs: Giantresources for the 21st century
Unconventional hydrocarbon resources in China and the prospect of exploration and development
Formation, distribution, potential and prediction of global conventional and unconventional hydrocarbon resources
“Exploring petroleum inside source kitchen”: Connotation and prospects of source rock oil and gas
Formation mechanism, geological characteristics and development strategy of nonmarine shale oil in China
Recent progress and prospect of oil and gas exploration by PetroChina Company Limited
Connotation and strategicrole of in-situ conversion processing of shale oil underground in theonsh onshore China
Selection of pilot areas for testing in-situ conversion/upgrading processing in lacustrine shale: A case study of Yanchang-7 member in Ordos Basin
Classification and evaluation criteria of shale oil and gas resources: Discussion and application
Oil content in argillaceous dolomite from the Jianghan Basin, China: Application of new grading evaluation criteria to study shale oil potential
A comparison of experimental methods for describing shale pore features: A case study in the Bohai Bay Basin of eastern China
Analysis of chemical reaction kinetics using a distribution of activation energies and simpler models
A chemical kinetic model of vitrinitematuration and reflectance
Global kinetic analysis of complex materials
Simple kinetic models of petroleum formation. Part I: Oil and gas generation from kerogen
Thermal cracking of kerogen in open and closed systems: Determination of kinetic parameters and stoichiometric coefficients for oil and gas generation
Modeling petroleum formation from heterogeneous source rocks: The influence of frequency factors on activation energy distribution and geological prediction
Hydrocarbon generation kinetics of lacustrine Yanchang shale in southeast Ordos Basin, North China
Hydrocarbon generation kinetics of a heterogeneous source rock system: Example from the lacustrine Eocene-Oligocene Shahejie Formation, Bohai Bay Basin, China
Quick evaluation of source rock kerogen kinetics using hydrocarbon programs from regular Rock-Evalanalysis
A numerical method for calculating total oil yield using a single routine Rock-Eval program: A case study of the Eocene Shahejie Formation in Dongying Depression, Bohai Bay Basin, China
Shale in-situ mining technology status quo of challenges and opportunities
Converting oil shale to liquid fuels: Energy inputs and greenhouse gas emissions of the shell in situ conversion process
Oil shale test project: Oil shale research and development project. Texas: Shell Frontier Oil and Gas