Introduction
Coal-derived gas plays a major role in the natural gas industry in China. By 2018, the total reserves of coal-derived gas accounted for 60.1% of total proved natural gas reserves, while giant coal-derived gas fields made up 50.93% of total natural gas production in China[1,2]. In addition to middle-shallow coal-derived gases generated from low-mature and mature source rocks, there are abundant highly and over mature coal-derived gas resources located in deep basins in China, such as Kela 2 gas field in the Kuche Depression of the Tarim Basin, Xushen gas field in the Xujiaweizi Fault Depression of the Songliao Basin and newly discovered Qingyang gas field in southwestern Ordos Basin[3]. However, there still exist some gaps in the knowledge about coal-derived gas generation at highly to over mature stage of source rocks, which has limited the accurate evaluation of resource potential of deep-seated coal-derived gases at this stage[4]. The hydrocarbon generation mechanisms involving interactions between organic and inorganic matters have been a hot topic in academic research in recent years. The roles of water and inorganic minerals in hydrocarbon generation and expulsion are attracting increasing attention[5,6,7,8,9,10,11,12,13,14]. The interactions between formation water and organic matters and their contribution to hydrocarbon generation got the most extensive investigation in this area, since water may provide hydrogen for hydrocarbon generation[6,14-19]. It has been demonstrated that water could be involved in thermal cracking reactions of organic matters, providing hydrogen for hydrocarbon generation, and enhancing yields of liquid saturated hydrocarbon[16,17,18]. Nowadays, the enhancement effect of water on petroleum generation has been widely accepted. However, it is still not clear regarding the effect of water on natural gas generation[20,21]. The results of some experiments illustrated that water had a negative effect on hydrocarbon gas generation from mature to highly mature organic matters[6, 16-18]. Wang et al.[15, 20, 22] revealed that water had no significant effect on hydrocarbon gas generation from mature to highly mature humic type organic matters. The water did not show similar enhancement effect on hydrocarbon gas generation from mature to highly mature organic matters in previous experiments. In addition, previous studies mainly focused on the effect of water on hydrocarbon gas generation during mature to highly mature stage of organic matters. The role of water in natural gas generation from over mature organic matters, however, has been rarely investigated. Highly to over mature coal-measure source rocks are depleted in hydrogen and enriched in carbon due to the organic matter type and high maturity. The hydrogen-bearing formation water, therefore, may be of great significance in hydrocarbon generation for highly to over mature coal-measure source rocks. So it is necessary to reveal the effect of water on natural gas generation from highly to over mature coal-measure source rocks to have a better understanding of the formation mechanism and resource potential of deep-seated coal-derived gases. In this study, thermal simulation experiments under high temperature and pressure were conducted to investigate the effect of water on gas generation from highly to over mature humic organic matters. Then the significance of water for the generation of deep-seated highly to over mature coal-derived gases was discussed based on the experimental results.
1. Material and experiments
1.1. Material
The coal sample used was collected from the Lower Cretaceous Shahezi Formation in the Songliao Basin. The coal-bearing strata in the Shahezi Formation are the major source rocks for deep-seated natural gases in the Songliao Basin. The coal sample has a vitrinite reflectance (Ro) of 0.62% and a total organic carbon (TOC) of 63.6%. The amounts of free hydrocarbon, pyrolysed hydrocarbon and pyrolysed carbon dioxide are of 0.92 mg/g, 120 mg/g and 3.6 mg/g, respectively. The maximum value of pyrolysis temperature is 422 °C. Hydrogen index and oxygen index are 187 mg/g and 6 mg/g, respectively.
There existing some free water and bound water in the coal. In order to reduce the influence of the water on experimental results, the coal sample was crushed to pieces with size less than 0.12 mm and dried at 100 °C for 2 h to evaporate the free water. Water used in this study was got from the Chinese Academy of Geological Science. It is a national standard material (No. GBW(E)070016 ) with a δ2H value of -4.8‰.
1.2. Pyrolysis experiment
The pyrolysis experiments were conducted with a gold capsule thermal simulation system in the Petroleum Geology Research and Laboratory Center of PetroChina Research Institute of Petroleum Exploration and Development. There are two batches of experiments, one with coal only and one with coal and water. The coal samples were heated for 72 h at specific temperature and pressure under conditions with and without water. The pressure was kept at 40 MPa during the pyrolysis to simulate the formation pressure, and the temperature was set at 400, 430, 450, 500 and 550 °C, respectively to simulate the high mature environment. Then the yields and characteristics of gaseous products from experiments with and without water (with same pyrolysis temperatures) were compared to investigate the effect of water on gas generation from humic type organic matters at highly to over mature stage.
The process of pyrolysis experiment includes capsule making, sample loading, capsule sealing, leakage detecting and sample heating. First, gold capsules with a uniform size (50 mm length, 5.5 mm o.d., 0.5 mm wall thickness) were made, and one end of the capsules was sealed with argon-arc welding. Then coal samples were loaded into gold capsules. For experiments with water, the water was injected into capsules after the loading of coal. And then the air in the capsules was expelled by argon, and the open end of capsules was sealed by argon-arc welding. After that, the sealed capsules were immersed in hot water (80 °C) for leakage detecting. The absence of bubbles out from gold capsules indicated a good sealing. At last, the gold capsules loaded with samples were heated in autoclaves. There were two gold capsules in each autoclave with the same temperature, one with coal only and the other one with coal and water. Heating of two capsules in the same autoclave could ensure the same pyrolysis condition for two capsules. The pressure in the autoclave was kept at 40 MPa with a high-pressure water pump. The temperature in the autoclave was increased from room temperature to the desired temperature at 20 °C/h, and held for 72 h at the target temperature. Then the autoclaves were cooled down to room temperature by circulating water. After cooling, the capsules were taken out for analysis.
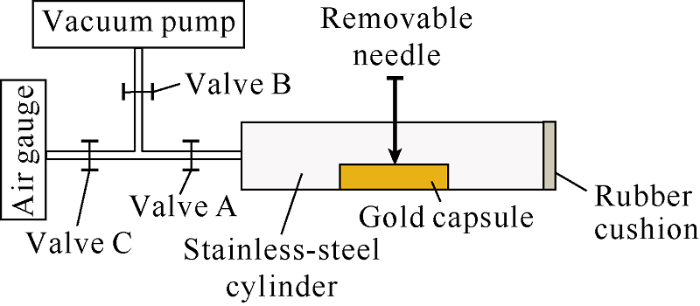
The gold capsule was put into a stainless-steel cylinder with a specific volume (Fig. 1). Then the cylinder was pumped to a near-vacuum state, the pressure of p1 (less than 1×10-9 Pa) was recorded. After that, the capsule was pierced, and the pressure in the cylinder would increase to p2 due to the release of gases from the capsule. The total volume of gaseous pyrolytic products (V) was calculated with the equation (1)[23]:
where, V0—volume of the gas collection system, cm3; p1, p2—pressures measured in the system, Pa; V—volume of gaseous pyrolytic products, cm3; p0—atmospheric pressure, Pa.
Fig. 1.
Fig. 1.
The sketch of gas collection device in hydrocarbon-generation thermal simulation experiment.
After the determination of the total volume of gaseous products, gases were extracted from the closed gas collection device for composition and carbon and hydrogen isotopic analysis. The compositional analysis was conducted on an Agilent 7890 gas chromatograph which was equipped with a Poraplot Q column (30 m×0.25 mm×0.25 μm) and used helium as the carrier gas. The GC was calibrated with an external standard and the accuracy was less than 1% in relative errors. Stable carbon isotopic compositions were determined with Isochrom II GC-IRMS. The GC was equipped with a Poraplot Q column, and the helium was used as the carrier gas. Analytical error for three times of analysis was less than 0.3‰.
Stable hydrogen isotopic compositions were determined with GC/TC/IRMS. The mass spectrometer used in the system was the MAT 253. Gases were separated on a PLOT Q column (30 m×0.32 mm×20 μm) with helium as the carrier gas. Analytical error for three times of analysis was less than 3‰.
2. The results
2.1. Yields of gaseous products
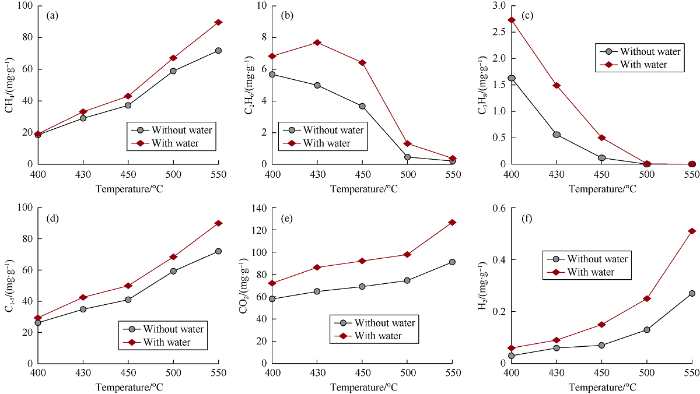
The pyrolytic products in experiments with and without water are similar in chemical composition, dominated by hydrocarbon gases and CO2 and containing small amounts of H2. In addition, there are tiny amounts of H2S generated from pyrolysis experiments at relatively low temperatures (Table 1). Hydrocarbon gases are dominated by methane. The yields of methane increase consistently with pyrolytic temperature in both series of the experiments (Fig. 2a), indicating the increase of cumulative methane yield with increasing coal maturity. Methane yields in experiments with water are higher than those in experiments without water at all temperatures (Fig. 2a). But the yield difference is very small at the pyrolytic temperature of 400 °C (Ro of 1.75%). Instead, there are significant differences of methane yield between two series of experiments when pyrolytic temperatures reach 430 °C (Ro of 2.27%). With the addition of water, methane yields increased by 13.9% and 24.9% at 430 °C (Ro of 2.27%) and 550 °C (Ro of 4.34%), respectively, implying that water could significantly enhance hydrocarbon generation from humic type organic matters at over mature stage. The yields of ethane decrease consistently with pyrolytic temperature in experiments with coal only (Fig. 2b). Ethane yields in experiments with coal and water, however, firstly increase and then decrease with the pyrolytic temperature, reaching the peak yield at 430°C (Fig. 2b). The yields of propane in both series of experiments decrease consistently with pyrolytic temperature (Fig. 2c). The decrease of ethane and propane yields with increasing temperature was caused by the thermal cracking. The yields of ethane and propane in experiments with coal and water are slightly higher than those in experiments without water at all temperatures (Fig. 2b, 2c). However, the yield differences between two series of experiments are small at high temperatures due to thermal cracking of ethane and propane. The yields of total hydrocarbon gases in experiments with water are higher than those in experiments without water at all pyrolytic temperatures (Fig. 2d). But the enhancement of water on total hydrocarbon gas yield is significant only at pyrolytic temperatures higher than 430 °C (Ro of 2.27%), which indicated that water had a significant promoting effect on hydrocarbon gas generation from humic type organic matters at over mature stage.
Table 1 The yields and carbon and hydrogen isotopic compositions of gaseous products formed in pyrolysis experiments.
| Series | Temperature/°C | Coal/mg | Water/mg | Ro/% | Absolute yield/(mg·g-1) | Mole ratio of C1 and C1-5 | δ13C/‰ | δ2H/‰ | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CO2 | CH4 | C2H6 | C3H8 | C4H10 | C5H12 | H2 | H2S | C1-5 | CO2 | CH4 | C2H6 | C3H8 | CH4 | C2H6 | ||||||
| Without water | 400 | 120.9 | 1.75 | 57.91 | 18.61 | 5.68 | 1.63 | 0.28 | 0.03 | 0.03 | 0.03 | 26.23 | 0.83 | -16.8 | -30.2 | -22.5 | -20.5 | -300.5 | -208.2 | |
| 430 | 104.5 | 2.27 | 64.68 | 29.11 | 4.99 | 0.56 | 0.06 | 0.12 | 0.06 | 0.02 | 34.83 | 0.91 | -16.5 | -28.4 | -19.6 | -13.7 | -278.4 | -140.2 | ||
| 450 | 79.0 | 2.66 | 69.01 | 37.22 | 3.67 | 0.12 | 0.07 | 41.01 | 0.95 | -15.9 | -27.0 | -15.7 | -6.2 | -254.0 | -124.4 | |||||
| 500 | 60.0 | 3.64 | 74.53 | 58.81 | 0.46 | 0.13 | 59.27 | 1.00 | -15.0 | -24.1 | -8.9 | -14.5 | -180.8 | -148.0 | ||||||
| 550 | 49.8 | 4.34 | 91.04 | 71.77 | 0.21 | 0.27 | 71.98 | 1.00 | -16.2 | -22.4 | -14.4 | -16.6 | -164.1 | -148.0 | ||||||
| With water | 400 | 122.4 | 25.3 | 1.75 | 72.09 | 19.23 | 6.83 | 2.73 | 0.57 | 0.07 | 0.06 | 0.06 | 29.42 | 0.80 | -16.7 | -30.2 | -22.7 | -20.9 | -282.4 | -204.3 |
| 430 | 102.4 | 20.0 | 2.27 | 86.30 | 33.17 | 7.69 | 1.49 | 0.16 | 0.02 | 0.09 | 0.09 | 42.52 | 0.88 | -16.5 | -28.1 | -20.9 | -16.5 | -268.2 | -131.3 | |
| 450 | 79.1 | 16.7 | 2.66 | 92.03 | 43.01 | 6.42 | 0.50 | 0.02 | 0.15 | 49.95 | 0.92 | -17.2 | -27.0 | -18.1 | -9.3 | -247.2 | -105.4 | |||
| 500 | 62.1 | 12.7 | 3.64 | 97.83 | 67.10 | 1.31 | 0.01 | 0.25 | 68.43 | 0.99 | -15.4 | -23.8 | -5.7 | -12.6 | -168.7 | -111.0 | ||||
| 550 | 51.2 | 10.0 | 4.34 | 126.70 | 89.61 | 0.38 | 0.51 | 89.99 | 1.00 | -16.5 | -22.1 | -11.9 | -13.2 | -135.4 | -123.0 | |||||
Fig. 2.
Fig. 2.
The yields of gaseous products from pyrolysis experiments with and without water.
Non-hydrocarbon gaseous products are dominated by CO2, and contain small amounts of H2. The yields of both CO2 and H2 increase consistently with temperature in both series of experiments (Fig. 2e, 2f). Experiments with water yielded more CO2 and H2 than experiments without water at the same temperature (Fig. 2e, 2f). The observation indicates that water has a significant enhancement on CO2 and H2 generation from highly to over mature humic type organic matters, which is consistent with previous studies[6, 15].
2.2. Carbon and hydrogen isotopic compositions of gaseous products
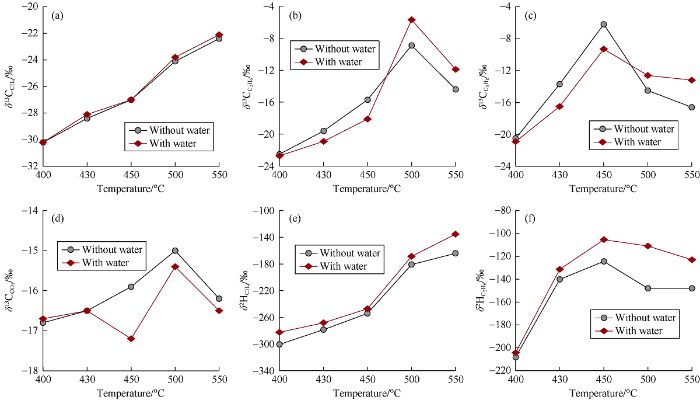
The carbon and hydrogen isotopic compositions of hydrocarbon gases and carbon isotopic compositions of CO2 in pyrolysis experiments were listed in Table 1. The δ13C values of methane in both series of experiments increase consistently with pyrolytic temperature (Fig. 3a), indicating an increase of δ13CCH4 with the maturity of source rock. There is no obvious difference of δ13CCH4 between experiments with and without water at each temperature (Fig. 3a), which indicates that addition of water did not have a significant effect on carbon isotopic compositions of methane. The values of carbon isotopic of ethane and propane reversed at high temperatures and resulted in partial carbon isotopic composition reversal of hydrocarbon gases (δ13C1<δ13C2>δ13C3) at 500 °C and 550 °C in both series of experiments (Fig. 3b, 3c). This situation is usually observed in highly to over mature coal-derived gases located in deep basins[3]. There is no regularity in carbon isotopic composition difference of ethane and propane between two series of experiments (Fig. 3b, 3c). According to the above experimental results, it can be inferred that water did not have a significant effect on carbon isotopic compositions of hydrocarbon gases generated from highly to over mature humic type organic matters. Wang et al.[15, 22] found that the addition of water also did not significantly change carbon isotopic compositions of hydrocarbon gases generated from mature to highly mature humic type organic matters. Therefore, it can be inferred that carbon isotopic compositions of coal-derived gases are mainly determined by organic matter composition and maturity, with rare influence from formation water. Carbon isotopic compositions of CO2 in both series of experiments have no systematic change with temperature. In addition, the differences of δ13CCO2 between two series of experiments are small and random (Fig. 3d).
Fig. 3.
Fig. 3.
Carbon and hydrogen isotopic compositions of gaseous products from pyrolysis experiments.
The δ2H values of methane in both series of experiments increase consistently with temperature (Fig. 3e), reflecting an increase of hydrogen isotopic composition with the increasing source rock maturity. The δ2H values of ethane firstly increase and then decrease with pyrolytic temperature, reaching the peak value at 450 °C (Fig. 3f), which indicating the hydrogen isotopic rollover in both series of experiments. δ2H values of methane and ethane in experiments with water are slightly higher than those in experiments without water at all temperatures (Fig. 3e, 3f), which indicating that the addition of 2H-enriched water resulted in heavier hydrogen isotopic composition in hydrocarbon gases generated from humic type organic matters during highly to over mature stage.
It is notable that carbon and hydrogen isotopic rollover of heavy hydrocarbon gases occurred in both series of experiments, and caused partial carbon isotopic composition reversal of hydrocarbon gases. This result indicates that carbon isotopic reversal of highly to over mature coal-derived gases is not caused by interactions between formation water and organic matters. It has been demonstrated that isotopic reversal of highly to over mature coal-derived gases may be derived from the cracking of straight-chain organic matter entrapped in kerogen at high temperature[24].
3. Discussion
3.1. Enhancement of water on gas generation from coal-measure source rock at over mature stage
Some researchers have investigated the effect of water on natural gas generation at mature to highly mature stage of source rocks[6, 16-18, 20]. The results indicated that water had an insignificant or retarding effect on hydrocarbon gas generation during mature to highly mature stage of source rocks. The reason for the retardation of water on hydrocarbon gas generation may be that water-derived hydrogen atoms combined with free-radical sites on high-molecular-weight hydrocarbon products (liquid hydrogen), which would retard secondary cracking reactions. However, in this study, we found that water could significantly enhance hydrocarbon gas generation during over mature stage of coal-measure source rock. So the effect of water on hydrocarbon gas generation from humic type organic matters is most likely controlled by the maturity of organic matters. There are two possible mechanisms for the enhanced hydrocarbon gas yields in experiments with water at temperatures higher than 430 °C: (1) catalytic decomposition of organic matters in supercritical water; (2) water provided external hydrogen for hydrogen-depleted humic type organic matters at over mature stage, which could enhance gas generation potential of organic matters.
Our pyrolysis experiments were conducted under 400- 550°C and 40 MPa, exceeding the supercritical point of water (374 °C, 22.05 MPa). So the water was presented with a supercritical state in experiments. Zhang et al.[25] investigated the reaction mechanisms of coal pyrolysis in supercritical water with ReaxFF reactive force field and the density functional theory (DFT) method, and demonstrated that there exist water clusters in supercritical water. These water clusters could reduce the cracking energy of C-C bonds in aromatic rings, and therefore facilitate aromatic ring-opening reactions. The linear chains hydrocarbon generated from aromatic ring-opening reaction would be further decomposed to small molecular gases, such as H2, CO and hydrocarbon gases. Supercritical water served as both catalyst and reactant in aromatic ring-opening reactions and following cracking of linear chains hydrocarbon. This effect of supercritical water on coal pyrolysis is a potential mechanism for the enhanced hydrocarbon gas yields in experiments with water. However, the gaseous products generated from catalytic decomposition of coal in supercritical water are dominated by H2 and CO. No CO was detected in pyrolytic products, and yields of H2 were very low in our experiments. So it can be inferred that catalytic decomposition of organic matters in supercritical water is not the primary mechanism for enhanced hydrocarbon gas yields in experiments with water. Besides, the addition of water at 400 °C (Ro of 1.75%) did not have a significant effect on hydrocarbon gas yields, indicating that the special properties of supercritical water were not responsible for enhanced hydrocarbon gas yields mentioned above.
Seewald[8] suggested that the involvement of water-derived hydrogen in thermal cracking of organic matters might significantly enhance the hydrocarbon gas generation from organic matters with high maturities. However, this proposition has not been verified experimentally, which may be due to the relatively low maturity of organic matters in previous pyrolysis experiments. This study revealed that water had a significant enhancement on hydrocarbon gas generation from coal when the coal got into over mature stage. The reason for the enhancement is that hydrogen content in organic matters is the primary limiting factor for hydrocarbon generation potential when kerogen gets into over mature stage and the involvement of external hydrogen at this stage will significantly improve the hydrocarbon potential of organic matters. During relatively low mature stage of organic matters, the effect of external hydrogen on hydrocarbon gas generation may be insignificant or obscured by other reactions due to the high hydrogen content in organic matters. But when the organic matters get into over mature stage with hydrogen-deficient characteristic, the external hydrogen will promote hydrocarbon generation. Hydrocarbon gases generated in experiments with water have higher δ2H values than those in experiments without water, which indicates a contribution of hydrogen from 2H-enriched water (δ2H=-4.8‰) in hydrocarbon gases. Therefore, it can be inferred that water could provide external hydrogen for hydrocarbon generation during highly to over mature stage of humic type organic matters and significantly enhance hydrocarbon gas generation from over mature humic type organic matters.
The addition of water in pyrolysis experiments also increased yields of CO2 and H2 significantly. CO2 was mainly derived from decarboxylation of organic matters during pyrolysis. The enhancement of water on CO2 generation was also observed in previous pyrolysis experiments conducted at relatively low temperatures of less than or equal to 370 °C[9, 15-18]. Some researchers found that oxygen in CO2 generated from organic matters exceeded oxygen loss from organic matters in hydrous pyrolysis, providing compelling evidence for the involvement of water-derived oxygen in CO2 generation[17,26-27]. There are several mechanisms through which water was involved in CO2 generation and enhanced CO2 yields: (1) Reactions between water and carbonyl groups in organic matters. Lewan et al.[17] suggested that water could react with aldehydes, esters and ketones to form carboxyl groups. These carboxyl groups could further decompose to CO2 through decarboxylation reactions. (2) Reactions between water and dissolved alkanes. Seewald[8] suggested that water could react with dissolved hydrocarbon to form carboxylic acids through intermediate products like alcohols and ketones. The carboxylic acids could decompose to generate CO2 ultimately. In the reactions mentioned above, water-derive oxygen was combined with organic matters to generate CO2, causing the release of water-derived hydrogen. These released hydrogen atoms could incorporate into H2 or combine with free-radical sites on alkyls and high-molecular-weight organic matters. Through this way, water-derived hydrogen can be involved in hydrocarbon generation as external hydrogen and increase hydrocarbon generation potential of organic matters.
In summary, the results of pyrolysis experiments indicate that water could be involved in hydrocarbon generation from humic type organic matters as an external hydrogen source during highly to over mature stage. The involvement of water could significantly enhance hydrocarbon gas generation during over mature stage of humic type organic matters, and then increase the hydrocarbon potential of over mature coal-measure source rocks.
3.2. Factors affecting hydrogen isotopic compositions of highly to over mature natural gases
It has been demonstrated that hydrogen isotopic compositions of natural gases are primarily controlled by depositional environment and thermal maturity of source rocks[28,29,30]. Source rock maturity is the main factor affecting hydrogen isotopes of generated gases when the depositional environment is similar. The control of source rock maturity on hydrogen isotopic compositions of natural gases is caused by hydrogen isotopic kinetic fractionation during gas generation[29, 31]. C-C bonds bounded with 1H atoms are easier to break than those bounded with 2H atoms. C-C bonds bonded with more 2H atoms have higher cracking energies. So alkyls containing less 2H atoms are more easily to be released during thermal cracking of organic matters. Hydrogen isotopic composition of generated hydrocarbon gases tends to be heavier during thermal maturation of organic matters. In other words, hydrogen isotopic composition of generated hydrocarbon gases will increase with maturity of source rocks. Hydrogen isotopic compositions of hydrocarbon gases were rarely reported in previous investigations regarding the effect of water on gas generation from organic matters. Wang et al.[15, 20, 22] found that addition of 2H-enriched water in coal pyrolysis caused lighter hydrogen isotopic composition of hydrocarbon gas generated from low mature to mature humic type organic matters. Our study, instead, showed that addition of 2H-enriched water could lead to heavier hydrogen isotopic composition of generated hydrocarbon gas during highly to over mature stage of humic type organic matters. The different effects of water on hydrogen isotopic compositions of hydrocarbon gases further indicate that influence of water on gas generation from humic type organic matters and product characteristics is controlled by maturity of organic matters. However, the water did not obscure the relationship between hydrogen isotopes of hydrocarbon gas and organic matter maturity, which was caused by isotopic kinetic fractionation. Therefore, we believe that assuming a similar depositional environment for source rocks, hydrogen isotopic compositions of highly and over mature coal-derived gases are controlled primarily by source rock maturity, with secondary influence from formation water. There are different possible mechanisms for the influence of formation water on hydrogen isotopic compositions of hydrocarbon gas. For instance, water-derived hydrogen atoms may be added into hydrocarbon gases directly by combining with the free-radical sites on alkyls (methyl, methylene, ethyl, etc.) generated from thermal cracking of organic matters. Also, water-derived hydrogen could get into high-molecular-weight organic matters through radical reactions or hydrogen exchange reactions and then get into hydrocarbon gases through cracking[31,32,33].
3.3. Geological implication
Highly and over mature coal-measure source rocks located in deep strata are widely distributed in China, such as Lower Permian coal-measure strata in the Ordos Basin, Carboniferous and Permian coal-measure strata in the Bohaiwan Basin, Triassic and Jurassic coal-measure strata in the Kuche Depression of the Tarim Basin and Lower Cretaceous coal-measure strata in the Songliao Basin. The Ordos Basin has the highest natural gas production in China. Carboniferous and Permian coal-measure source rocks are distributed throughout the whole basin and have higher burial depths and maturities in the southern part. They are in over mature stage and have Ro up to 2.8% along the Yanan-Wuqi region[1]. The Kuche Depression in the Tarim Basin is one of four major terrestrial gas-producing areas in China. Triassic and Jurassic coal-measure strata are the major source rocks for hydrocarbon gases in the depression. They are at highly to over mature stage and have Ro over 2.2% around the Baicheng area[1]. Lower Cretaceous coal- measure strata in the Songliao Basin are the main source rocks for hydrocarbon gases in deep fault-depressions. Organic matters in the source rocks are also in over mature stage. The Ro of organic matters could reach up to 3.6%, with an average value higher than 2.0%[34]. Carboniferous and Permian coal- measure source rocks deeply located in the Bohaiwan and southern North China Basin have Ro values over 5.0%[3].
The results of pyrolysis experiments in this study indicate that water could provide external hydrogen for gas generation during thermal cracking of coal at highly to over mature stage. The participation of water could enhance hydrocarbon gas generation from coal significantly at over mature stage. Water is ubiquitous in source rocks. It is mainly stored in inorganic and organic pores as irreducible water[10,11,12]. Although the water content in source rocks is low (generally less than 3%), the hydrogen contained in water still has great significance for hydrocarbon generation. The hydrogen supply from formation water may be able to significantly increase hydrocarbon generation potential of highly to over mature coal-measure source rocks and therefore has great implications for the assessment of over mature coal-derived gas resources. However, it still needs more work to reveal the specific contribution of formation water on natural gas generation from over mature coal- measure source rocks in real geological setting, since there are obvious differences in temperature, pressure and water content between pyrolysis experiment and real strata.
4. Conclusions
The results of pyrolysis experiments in this study indicate that water could be involved in thermal cracking of humic type organic matters at highly to over mature stage and provide hydrogen for hydrocarbon gas generation. The effect of water-derived hydrogen on yields and isotopic compositions of hydrocarbon gases was controlled by the maturity of organic matters. In the pyrolysis experiments, water could significantly enhance gas generation from humic type organic matters at over mature stage. The yields of hydrocarbon gases increased by 13% with the addition of water in experiments. In addition, the participation of 2H-enriched water in hydrocarbon gas generation could lead to heavier hydrogen isotopic composition of hydrocarbon gases generated from highly and over mature humic type organic matters. Although water showed significant enhancement on hydrocarbon gas generation from over mature coal in pyrolysis experiments, it still needs more work to figure out the specific contribution of formation water on natural gas generation from coal-measure source rocks in deep and ultra-deep strata, due to the great differences in hydrocarbon generation conditions between pyrolysis experiment and real geological strata. Our study provides a new aspect needed to be considered during assessment of deep and ultra-deep coal-derived gas resources.
Reference
The significance of coal-derived gas in major gas producing countries
Major progress in the natural gas exploration and development in the past seven decades in China
Discovery and geological knowledge of large deep coal-formed Qingyang Gas Field, Ordos Basin, NW China
Onshore deep and ultra-deep natural gas exploration fields and potentials in China
Gas generation and its isotope composition during coal pyrolysis: The catalytic effect of nickel and magnetite
DOI:10.1016/j.fuel.2018.02.118 URL [Cited within: 1]
Generation of hydrocarbon gases and CO2 from a humic coal: Experimental study on the effect of water, minerals and transition metals
DOI:10.1016/j.orggeochem.2005.12.004 URL [Cited within: 5]
Evaluating transition-metal catalysis in gas generation from the Permian Kupferschiefer by hydrous pyrolysis
DOI:10.1016/j.gca.2008.06.003 URL [Cited within: 1]
Organic-inorganic interactions in petroleum producing sedimentary basins
DOI:10.1038/nature02132
URL
PMID:14628062
[Cited within: 3]

Petroleum deposits form as a consequence of the increased temperatures that accompany progressive burial of organic matter deep within sedimentary basins. Recent advances in petroleum geochemistry suggest that inorganic sedimentary components participate in organic transformations associated with this process. Water is particularly important because it facilitates reaction mechanisms not available in dry environments, and may contribute hydrogen and oxygen for the formation of hydrocarbons and oxygenated alteration products. These findings suggest that petroleum generation and stability is influenced by subsurface chemical environments, and is a simple function of time, temperature and the composition of sedimentary organic matter.
Kerogen pyrolysis in the presence and absence of water and minerals. 1. Gas components
Water distribution in overmature organic-rich shales: Implications from water adsorption experiments
Water content and equilibrium saturation and their influencing factors of the lower Paleozoic overmature organic-rich shales in the Upper Yangtze Region of Southern China
Evolution of water content in organic-rich shales with increasing maturity and its controlling factors: Implications from a pyrolysis experiment on a water-saturated shale core sample
DOI:10.1016/j.marpetgeo.2019.06.023 URL [Cited within: 2]
The influences of maturation and water in the isotopic composition of pyrolysis
Water consumption in hydrocarbon generation and its significance to reservoir formation
DOI:10.1016/S1876-3804(13)60029-4 URL [Cited within: 2]
Pyrolytic simulation experiments on the role of water in natural gas generation from coal
DOI:10.1016/j.coal.2008.02.003
URL
[Cited within: 5]

Abstract
In order to probe into the role of water in the process of natural gas generation, lignite from the Shenshan coal field of the Ordos Basin, China, was selected for pyrolytic simulation experiments under hydrous and anhydrous conditions for the purpose of comparison. The results showed that water was involved in the chemical reactions that took place during gas generation, and enhanced the productivity of CO2 and H2. Moreover, the involvement of water led to an increase in the productivity of hydrocarbons in a certain degree, especially methane at high temperatures. The δD values of CH4 become linearly heavier with increasing productivity of methane as observed in the two series of experiments, and the δD values of CH4 in the hydrous experiments are about 35‰ lighter than those obtained in the anhydrous experiments in the case of the same productivity of CH4. The fact that the δ13C values of CO2 have become lighter in the hydrous experiments is strong evidence for the involvement of water in the chemical reactions during gas generation. Such trend of variations appears to have been caused by the conversion of methylene carbon to carboxyl carbon by water/alkene interaction in hydrous condition.
Role of water in hydrocarbon generation from Type-I kerogen in Mahogany oil shale of the Green River Formation
DOI:10.1016/j.orggeochem.2010.10.004 URL [Cited within: 3]
Experiments on the role of water in petroleum formation
DOI:10.1016/S0016-7037(97)00176-2 URL [Cited within: 3]
Comparison of artificial maturation of lignite in hydrous and nonhydrous conditions
DOI:10.1016/S0146-6380(02)00241-3 URL [Cited within: 4]
The effect of water medium on the products of different pyrolysis system
DOI:10.11764/j.issn.1672-1926.2015.03.0524
URL
[Cited within: 1]

In order to further clarify the role of water in the evolution of organic matter,we summarize the effect of water in different pyrolysis system.The development of hydrocarbon evolution and expulsion simulation experiment is open system→closed system→semi-open system.And at the same time,the medium of experiment has also changed from anhydrous to hydrous pyrolysis.In the above three pyrolysis systems,semi-open simulation system has been regarded as the process which is closer to the actual conventional geological evolution.As liquid water has different properties at different temperature and pressure,different state of liquid water will have different effects on the products of simulation experiment.For example,the temperature between room temperature and 100℃is called normal temperature water.High temperature liquid water ranges from 200℃ to 374.5℃.But after the critical temperature (T=374.5℃) and pressure (P=22.1MPa) point,the physical and chemical properties of water will have a big change.In general,in the closed system,the generation of gas hydrocarbon has a positive correlation with the added water,but when the amount of water has a percent between 20 and 50 on the rock sample,the yield of liquid hydrocarbon is the highest.But in the semi-open system,different states of water also have different properties.For example,the medium of high pressure water vapour improves the efficiency of gaseous hydrocarbon.And the critical water medium is conductive to the generation and preservation of liquid hydrocarbon.So clearly understanding how the liquid water affects pyrolysis products will have a significant implication.
Influences of water media on the hydrogen isotopic composition of natural gas/methane in the processes of gaseous hydrocarbon generation and evolution
DOI:10.1007/s11430-011-4195-0 URL [Cited within: 4]
Experimental evidence for formation water promoting crude oil cracking to gas
DOI:10.1007/s11434-012-5015-4 URL [Cited within: 1]
Role of water in hydrocarbon gas generation from organic matters: Evidence from pyrolysis experiments
Synthesis of hydrocarbon gases from four different carbon sources and hydrogen gas using a gold-tube system by Fischer-Tropsch method
DOI:10.1016/j.chemgeo.2013.03.016
URL
[Cited within: 1]

Several series of Fischer-Tropsch synthesis (FTS) experiments were conducted in a gold tube system with montmorillonite K-10 loaded with Fe3+ and Ni3+ as catalysts. Four different carbon sources: graphite, Na2CO3 solution (20%) and two types of CO2 gases with distinctive isotopic compositions were reduced in pure hydrogen gas at 400 degrees C and 50 MPa for 2-60 h. The experimental results showed that the FTS reaction between liquid carbon and H-2 could hardly occur. However, the reaction between gaseous phase carbon (CO2) and H-2 gas was easier than that between solid phase carbon (graphite) and H-2 gas; and the C-13 depleted CO2 is more reactive than the C-13 enriched CO2. Our results also show that the production of synthetic hydrocarbon gases from different carbon sources with H-2 depends largely on the phases, and structural and thermal stability of carbon sources. In a relatively short reaction time at 400 degrees C, the carbon isotope values of the synthesized alkane gases showed a full reversal trend with their molecular carbon numbers (delta C-13(1) > delta C-13(2) > delta C-13(3) > delta C-13(4).) However, with increasing reaction time, such a reversed isotopic distribution pattern disappeared. Our interpretation is that the final products were gradually replaced by cracking of the hydrocarbon products formed at the earlier stage of the synthesis process. Thus the C-13 depleted gas from the thermal cracking was mixed with the C-13 enriched residual gas, leading to the occurrence of a partial reversal or a normal isotopic distribution among C-1-C-4 alkane series (delta C-13(1) < delta C-13(2) < delta C-13(3) < delta C-13(4)), similar to the thermogenic alkane gases in nature. Under longer reaction time or/and higher reaction temperature (700 degrees C), hydrocarbon gases would crack and generate monatomic carbon.
The observed great discrepancy between the natural abiogenic gas and synthetic gas is likely due to the big difference in temperatures between geological settings and the laboratory experiment process. FTS experiments conducted under laboratory experimental condition are usually from low to high temperature and differ significantly from the abiogenic synthesis process for hydrocarbons in real geological settings, which is perceived as a cooling process from high to low temperature either under aqueous hydrothermal or volcanic intrusion conditions. Under certain (stable) geological temperature/pressure conditions, hydrocarbon gases generated might never suffer further decomposition, and thus might preserve a fixed "inverse" molecular isotopic fingerprinting as we observed in the laboratory. This has also been proven by a cooling FTS experiment. (C) 2013 Elsevier B.V.
Gas generation and its isotope composition during coal pyrolysis: Potential mechanism of isotope rollover
DOI:10.1016/j.fuel.2018.05.029 URL [Cited within: 1]
The effect of supercritical water on coal pyrolysis and hydrogen production: A combined ReaxFF and DFT study
DOI:10.1016/j.fuel.2013.01.064
URL
[Cited within: 1]

The reaction mechanism of coal pyrolysis and hydrogen production in supercritical water (SCW) was investigated using the molecular dynamic simulations via the reactive force field (ReaxFF) method combined with the density functional theory (DFT) method. Our calculations present that the water clusters in SCW weaken the C-C bonds in aromatic rings, thus the C(ring)-C(ring) bond cracking energy decreases as much as 287.3 kJ/mol and 94.6 kJ/mol compared with that in pure coal pyrolysis and in coal pyrolysis in vapor state, respectively. After the aromatic rings break into small cyclic structures, such as quaternary rings and ternary rings, the water clusters in SCW further weaken their C-C ring bonds to induce the small cyclic rings to open. During this process, the water clusters (without any radicals) in SCW turn into H radical-rich water clusters after providing OH radicals to the cyclic rings. This is the main source for the production of hydrogen molecules in SCW-coal system. The combination of H radicals produced by coal with water clusters in SCW is another pathway which forms H radical-rich water clusters. Under the catalysis of water molecules or clusters, H radical-rich water clusters decompose into H-2 and OH radicals. These OH radicals further bind with coal intermediates and result in the breaking of coal intermediates into smaller products. Therefore, the cooperative effects between SCW and coal form a virtuous circle, which greatly enhances the reaction rate of coal gasification, promotes the production of small molecules, and increases the yield of hydrogen. (C) 2013 Elsevier Ltd.
Water as an oxygen source for the production of oxygenated compounds (including CO2 precursors) during kerogen maturation
DOI:10.1016/0146-6380(94)90120-1 URL [Cited within: 1]
Laboratory and theoretical constraints on the generation and composition of natural gas
DOI:10.1016/S0016-7037(98)00000-3 URL [Cited within: 1]
The hydrogen and carbon isotopic composition of methane from natural gases of various origins
DOI:10.1016/0016-7037(80)90155-6 URL [Cited within: 1]
Fundamental studies on kinetic isotope effect (KIE) of hydrogen isotope fractionation in natural gas systems
DOI:10.1016/j.gca.2011.02.016
URL
[Cited within: 2]

Based on quantum chemistry calculations for normal octane homolytic cracking, a kinetic hydrogen isotope fractionation model for methane, ethane, and propane formation is proposed. The activation energy differences between D-substitute and non-substituted methane, ethane, and propane are 318.6, 281.7, and 280.2 cal/mol, respectively. In order to determine the effect of the entropy contribution for hydrogen isotopic substitution, a transition state for ethane bond rupture was determined based on density function theory (DFT) calculations. The kinetic isotope effect (KIE) associated with bond rupture in D and H substituted ethane results in a frequency factor ratio of 1.07. Based on the proposed mathematical model of hydrogen isotope fractionation, one can potentially quantify natural gas thermal maturity from measured hydrogen isotope values. Calculated gas maturity values determined by the proposed mathematical model using delta D values in ethane from several basins in the world are in close agreement with similar predictions based on the delta(13)C composition of ethane. However, gas maturity values calculated from field data of methane and propane using both hydrogen and carbon kinetic isotopic models do not agree as closely. It is possible that dD values in methane may be affected by microbial mixing and that propane values might be more susceptible to hydrogen exchange with water or to analytical errors. Although the model used in this study is quite preliminary, the results demonstrate that kinetic isotope fractionation effects in hydrogen may be useful in quantitative models of natural gas generation, and that dD values in ethane might be more suitable for modeling than comparable values in methane and propane. (C) 2011 Elsevier Ltd.
Hydrogen isotopes of hydrocarbon gases from different organic facies of the Zhongbai gas field, Sichuan Basin, China
DOI:10.1016/j.petrol.2019.04.102 URL [Cited within: 1]
Hydrogen isotopic (D/H) composition of organic matter during diagenesis and thermal maturation
DOI:10.1146/annurev.earth.34.031405.125011 URL [Cited within: 2]
D/H isotope ratios of kerogen, bitumen, oil, and water in hydrous pyrolysis of source rocks containing kerogen types I, II, IIS, and III
DOI:10.1016/S0016-7037(99)00221-5 URL [Cited within: 1]
Hydrogen isotope exchange between n-alkanes and water under hydrothermal conditions
DOI:10.1016/j.gca.2011.10.008
URL
[Cited within: 1]

To investigate the extent of hydrogen isotope (H-2 and H-1) exchange between hydrocarbons and water under hydrothermal conditions, we performed experiments heating C-1-C-5 n-alkanes in aqueous solutions of varying initial H-2/H-1 ratios in the presence of a pyrite-pyrrhotite-magnetite redox buffer at 323 degrees C and 35-36 MPa. Extensive and reversible incorporation of water-derived hydrogen into C-2-C-5 n-alkanes was observed on timescales of months. In contrast, comparatively minor exchange was observed for CH4. Isotopic exchange is facilitated by reversible equilibration of n-alkanes and their corresponding n-alkenes with H-2 derived from the disproportionation of water. Rates of delta H-2 variation in C3+ n-alkanes decreased with time, a trend that is consistent with an asymptotic approach to steady state isotopic compositions regulated by alkane-water isotopic equilibrium. Substantially slower delta H-2 variation was observed for ethane relative to C-3-C-5 n-alkanes, suggesting that the greater stability of C3+ alkenes and isomerization reactions may dramatically enhance rates of H-2/H-1 exchange in C3+ n-alkanes. Thus, in reducing aqueous environments, reversible reaction of alkanes and their corresponding alkenes facilitates rapid H-2/H-1 exchange between water and alkyl-bound hydrogen on relatively short geological timescales at elevated temperatures and pressures. The proximity of some thermogenic and purported abiogenic alkane delta H-2 values to those predicted for equilibrium H-2/H-1 fractionation with ambient water suggests that this process may regulate the delta H-2 signatures of some naturally occurring hydrocarbons. (C) 2011 Elsevier Ltd.
A review of alkane gas geochemistry in the Xujiaweizi fault-depression, Songliao Basin
DOI:10.1016/j.marpetgeo.2013.01.009
URL
[Cited within: 1]

A large suite (172) of gases from the Yingcheng Fm. in the Xujiaweizi fault-depression has been studied chemically and isotopically. The results show that they are mainly type-III kerogen/coal-type gas and have been generated and accumulated under the influence of hydrothermal fluids. Both measured and calculated vitrinite reflectance data show that they have been generated mainly at source maturity higher than 2.0%Ro. Many gases show carbon isotopic reversals (typically, delta C-13(1) > delta C-13(2) and delta C-13(2) > delta C-13(3)). The origin of carbon isotopic reversals is discussed in this paper and the results show that previous interpretations, including (a) mixing and (b) the occurrence of abiogenic gases, are incorrect. Based on iso-butane/n-butane data, wet-gas cracking may have occurred but it is not the main cause of carbon isotopic reversals here. We propose that a process of abiogenic polymerization of C2+ gases with methane is the main cause of the carbon isotopic reversals in this basin. (C) 2013 Elsevier Ltd.