Introduction
The generation of natural gas runs through the whole thermal maturation process of organic matter, and the depth and temperature ranges of gas generation are much wider than those of oil generation[1,2,3,4,5,6]. Although after years of research, the theory of natural gas formation has made great progress, and the genetic identification has improved[7,8,9,10,11], new problems are emerging with the exploration targets gradually turning to deep, unconventional petroleum and complex tectonic hydrothermal activity areas[12]. For example, abnormal geochemical characteristics often occur in natural gas at high to over mature stages, including inversion of carbon isotopic compositions of alkane gas, reversal of carbon isotopic series, and even occurrence of negative carbon isotopic series of thermogenic gas[13,14,15,16,17]. Researchers have studied the "abnormal" geochemical phenomena of natural gas, and obtained a series of achievements. But there are still some controversies[15,16,17,18,19]. Some researchers believed that the "abnormal" carbon isotopic compositions of alkane gas was caused by water rock reaction[15, 19], while others held that the "anomalies" of carbon isotopic compositions of alkane gas were caused by the mixing of natural gases from different sources[15]. Since the gas reservoirs discovered all over the world are mostly formed by thermal maturation of organic matter, and thermal simulation experiment can dynamically investigate the whole process of gas generation in organic matter thermal maturation, in other words, it can reflect the isotopic composition characteristics in the process of natural gas generation. Therefore, thermal simulation experiment is considered as one of the most effective means to study isotopic fractionation mechanism in the process of natural gas generation[20,21,22,23,24,25,26,27,28]. In this study, the thermal simulation gas experiment of coal was used to analyze the parameter characteristics of natural gas composition, yield and carbon isotopic composition. Combined with results of previous studies, the mechanisms of carbon isotopic fractionation in the process of natural gas generation are discussed to further understand the formation mechanisms of natural gas and provide theoretical basis for natural gas exploration.
1. Samples and analysis
Among many thermal simulation experimental instruments, gold capsule thermal simulation system can simulate the influence of temperature and pressure on the thermal maturation of organic matter at the same time. Chemically inert, gold can prevent the catalytic effect of reaction device on hydrocarbon generation. Gold also has good ductility at high temperature, so gold capsule pyrolysis system is considered to be one of the most suitable systems to simulate gas generation characteristics of organic matter[22,23,24,25,26,27,28]. After comprehensive consideration, we select thermal simulation experiment of low mature coal in gold capsule to investigate the fractionation mechanism of carbon isotopic composition in the process of natural gas generation.
1.1. The samples
The sample was taken from the Lower Permian Shanxi Formation in the Shaping Coal Mine in the eastern Ordos Basin, NW China. The organic matter maturity of the sample was 0.55% (Ro%), the organic carbon content was 58.3%, the maximum pyrolysis temperature was 424 ºC, the free hydrocarbon content was 0.66 mg/g, the pyrolytic hydrocarbon content was 97.18 mg/g. The hydrogen index of the sample was 167 mg/g, which indicated that the organic matter of the sample was the gas-prone humic type. According to the standard of high quality gas source rock[3], the coal sample with hydrocarbon generation potential index (S1 + S2) of more than 60 mg/g was high quality gas source rock, the low mature coal sample in this study had much higher hydrocarbon generation potential index than 60 mg/g, so it had high gas generation potential.
1.2. The methods
The simulation device is the gold capsule closed experimental system, and the specific experimental method is presented in the references [24-25, 29]. Considering that the petroleum system often has high pressure at the high to over mature stages, the designed pressure of experiment was high. The pressure of thermal simulation experiment system was maintained at 50 MPa by using pressure sensor. In order to investigate the gas generation process of coal more comprehensively, 16 temperature points were designed in the experiment, with 320 ºC as the first temperature point and 650 ºC as the highest temperature point. The heating program was set as two modes, rapid heating (20 ºC/h) and slow heating (2 ºC/h). The thermal simulation experiment and product analysis were both done in the Key Laboratory of Petroleum Geochemistry, Research Institute of Petroleum Exploration and Development PetroChina.
2. The results
The parameters such as gas yield, component and carbon isotopic composition are shown in Tables 1 and 2.
2.1. Yield of the simulated gases
Through calculation of geochemical parameters such as the relative content of each component, total gas content, weight of sample and total organic carbon content, the yields of gas components in the thermal simulation can be obtained. The calculated yields of gas components can help assess the gas generation potential of the coal sample and the source rock. Through calculation, the yields of gas components of the coal sample are shown in Table 1.
Table 1 Yield of gas products of low mature coal sample from the Lower Permian Shanxi Formation in eastern Ordos Basin.
| Heating mode | Temperature/ ºC | Gas component yield/(mL•g-1) | Alkane gas yield/ (mL•g-1) | Heavy hydrocarbon gas yield/(mL•g-1) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CH4 | C2H6 | C3H8 | iC4H10 | nC4H10 | iC5H12 | nC5H12 | H2 | CO2 | H2S | ||||
| Rapid heating | 320 | 0.21 | 3.37 | 0.21 | |||||||||
| 340 | 0.62 | 0.02 | 3.68 | 0.64 | 0.02 | ||||||||
| 360 | 1.04 | 0.08 | 0.02 | 4.95 | 1.13 | 0.09 | |||||||
| 380 | 2.29 | 0.42 | 0.11 | 0.02 | 0.02 | 0.01 | 15.88 | 0.03 | 2.87 | 0.58 | |||
| 400 | 4.52 | 0.99 | 0.17 | 0.01 | 0.01 | 14.93 | 5.70 | 1.18 | |||||
| 420 | 11.02 | 3.34 | 0.75 | 0.04 | 0.06 | 26.41 | 15.20 | 4.18 | |||||
| 440 | 22.53 | 6.41 | 1.58 | 0.09 | 0.14 | 0.01 | 0.01 | 30.31 | 30.78 | 8.25 | |||
| 460 | 36.31 | 8.48 | 1.94 | 0.13 | 0.16 | 0.02 | 0.01 | 28.30 | 47.04 | 10.73 | |||
| 480 | 50.80 | 9.70 | 1.99 | 0.10 | 0.14 | 0.62 | 34.86 | 62.73 | 11.93 | ||||
| 500 | 64.36 | 10.27 | 1.60 | 0.07 | 0.07 | 1.06 | 41.15 | 76.38 | 12.02 | ||||
| 525 | 93.92 | 9.38 | 0.69 | 0.02 | 0 | 40.87 | 104.01 | 10.09 | |||||
| 550 | 135.4 | 6.49 | 0.33 | 0.06 | 0.10 | 0.04 | 0.04 | 1.50 | 51.01 | 142.44 | 7.04 | ||
| 575 | 167.14 | 2.27 | 1.83 | 54.51 | 169.40 | 2.27 | |||||||
| 600 | 194.15 | 1.08 | 2.10 | 49.31 | 195.24 | 1.08 | |||||||
| 625 | 229.41 | 0.75 | 0 | 2.59 | 48.27 | 230.16 | 0.75 | ||||||
| Slow heating | 320 | 0.45 | 0.04 | 0.01 | 6.22 | 0.50 | 0.05 | ||||||
| 340 | 1.22 | 0.15 | 0.02 | 9.65 | 1.40 | 0.18 | |||||||
| 360 | 3.93 | 0.93 | 0.17 | 0.01 | 0.01 | 16.31 | 5.05 | 1.11 | |||||
| 380 | 8.27 | 2.47 | 0.56 | 0.03 | 0.06 | 0.01 | 25.28 | 11.39 | 3.13 | ||||
| 400 | 20.89 | 5.41 | 1.14 | 0.05 | 0.10 | 0.01 | 0.01 | 28.11 | 27.61 | 6.71 | |||
| 420 | 31.76 | 7.15 | 1.55 | 0.08 | 0.14 | 0.01 | 0.01 | 34.28 | 40.70 | 8.94 | |||
| 440 | 44.67 | 7.72 | 1.35 | 0.08 | 0.09 | 0.42 | 36.69 | 53.91 | 9.24 | ||||
| 460 | 69.41 | 8.62 | 1.01 | 0.06 | 0.04 | 0.54 | 42.48 | 79.15 | 9.73 | ||||
| 480 | 89.91 | 6.98 | 0.38 | 0.02 | 1.36 | 43.69 | 97.28 | 7.37 | |||||
| 500 | 118.7 | 4.58 | 0.07 | 1.13 | 46.80 | 123.34 | 4.65 | ||||||
| 525 | 150.6 | 1.86 | 0.02 | 1.39 | 37.17 | 152.48 | 1.88 | ||||||
| 550 | 199.4 | 0.78 | 0.05 | 2.38 | 40.24 | 200.22 | 0.82 | ||||||
| 575 | 236.3 | 0.77 | 3.75 | 35.21 | 0.34 | 237.07 | 0.77 | ||||||
| 600 | 287.12 | 0.76 | 5.64 | 34.12 | 0.47 | 287.89 | 0.76 | ||||||
| 625 | 302.26 | 0.48 | 6.81 | 29.05 | 302.74 | 0.48 | |||||||
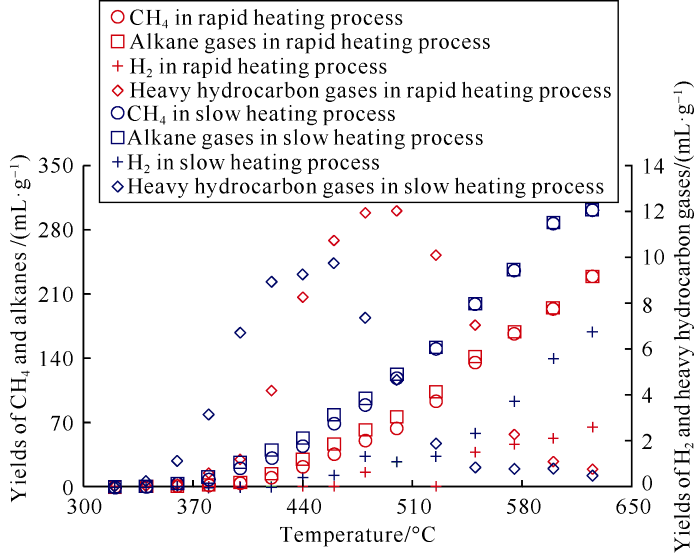
During the rapid and slow heating experiments, the maximum yields of CH4 were 229.41 ml/g and 302.26 ml/g, respectively, and the maximum yields of alkane gas were 230.16 ml/g and 302.74 ml/g, respectively (Fig. 1 and Table 1). At the same temperature, the alkane gas yield of the slow heating system was significantly higher than that of the rapid heating process. Under the condition of slow heating, the reactant reaction might be more sufficient, which was conducive to the decomposition of organic macromolecules into small molecules, and the yield of CH4 was significantly increased.
Fig. 1.
Fig. 1.
Yields of CH4, alkane gas, heavy hydrocarbon gas and H2 of the low mature coal samples from the Ordos Basin.
In the two groups of simulation experiments, the yields of heavy hydrocarbon gas were not high, the maximum values were 9.73 ml/g and 12.02 ml/g, and the corresponding temperatures were 460 °C and 500 °C respectively (Fig. 1 and Table 1). A small amount of H2 was detected at higher temperature points, and the yield of H2 increased with the increase of thermal simulation temperature (Fig. 1). In addition, a small amount of H2S was also detected in the thermal simulation gas products. Due to the low content and poor analysis accuracy of the device to H2S, the relationship between the H2S yield and temperature was not obvious; the CO2 yield generally increased with the increase of temperature, but its relative volume content gradually decreased with the increase of alkane gas (Table 1).
2.2. Components of the simulated gases
The components of hydrocarbon gases (CH4, C2H6, C3H8, iC4H10, nC4H10, iC5H12, nC5H12) and non-hydrocarbon gases (CO2, H2S, H2) in the simulated products at different temperatures were analyzed. The results are shown in Table 2.
Table 2 Relative contents of gas components produced and carbon isotopic compositions of low mature coal sample from the Lower Permian Shanxi Formation in the eastern Ordos Basin.
| Heating mode | Temperature/ ºC | Main gas components /% | Dryness coefficient/% | δ13C/‰ | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CH4 | C2H6 | C3H8 | iC4H10 | nC4H10 | iC5H12 | nC5H12 | H2 | CO2 | H2S | CH4 | C2H6 | C3H8 | iC4H10 | nC4H10 | |||
| Rapid heating | 320 | 5.95 | 94.05 | 100.00 | |||||||||||||
| 340 | 14.27 | 0.52 | 85.21 | 96.47 | |||||||||||||
| 360 | 17.07 | 1.25 | 0.29 | 81.39 | 91.75 | ||||||||||||
| 380 | 12.18 | 2.26 | 0.60 | 0.08 | 0.11 | 0.03 | 0.02 | 84.54 | 0.17 | 79.66 | -32.9 | ||||||
| 400 | 21.89 | 4.80 | 0.84 | 0.05 | 0.06 | 72.37 | 79.22 | -33.5 | -28.0 | -25.8 | |||||||
| 420 | 26.48 | 8.02 | 1.79 | 0.09 | 0.14 | 63.48 | 72.49 | -34.8 | -27.5 | -25.4 | -25.3 | -25.4 | |||||
| 440 | 36.88 | 10.50 | 2.58 | 0.15 | 0.23 | 0.02 | 0.02 | 49.61 | 73.20 | -34.8 | -26.2 | -24.8 | -25.8 | -25.1 | |||
| 460 | 48.19 | 11.25 | 2.58 | 0.17 | 0.21 | 0.02 | 0.01 | 37.56 | 77.19 | -33.9 | -25.5 | -23.9 | -25.1 | -24.0 | |||
| 480 | 51.72 | 9.88 | 2.02 | 0.11 | 0.14 | 0.64 | 35.49 | 80.97 | -32.2 | -24.6 | -23.0 | -23.6 | -21.8 | ||||
| 500 | 54.27 | 8.66 | 1.35 | 0.06 | 0.06 | 0.89 | 34.70 | 84.26 | -31.2 | -23.5 | -20.9 | ||||||
| 525 | 64.82 | 6.47 | 0.47 | 0.02 | 28.21 | 90.30 | -30.2 | -20.3 | -14.7 | ||||||||
| 550 | 69.45 | 3.33 | 0.17 | 0.03 | 0.05 | 0.02 | 0.02 | 0.77 | 26.16 | 95.06 | -27.9 | -15.6 | -18.8 | ||||
| 575 | 74.04 | 1.00 | 0.81 | 24.15 | 98.66 | -26.3 | -9.0 | ||||||||||
| 600 | 78.72 | 0.44 | 0.85 | 19.99 | 99.45 | -24.6 | -12.4 | ||||||||||
| 625 | 81.64 | 0.27 | 0.92 | 17.18 | 99.67 | -24.0 | -14.9 | ||||||||||
| 650 | -23.6 | -17.5 | |||||||||||||||
| Slow heating | 320 | 6.68 | 0.63 | 0.14 | 92.55 | 89.67 | |||||||||||
| 340 | 11.07 | 1.37 | 0.22 | 87.34 | 87.43 | -32.7 | -28.9 | -26.4 | |||||||||
| 360 | 18.42 | 4.34 | 0.78 | 0.03 | 0.06 | 76.36 | 77.92 | -34.8 | -28.3 | -25.4 | |||||||
| 380 | 22.55 | 6.74 | 1.53 | 0.07 | 0.15 | 0.01 | 0.02 | 68.93 | 72.57 | -34.8 | -27.5 | -25.2 | |||||
| 400 | 37.49 | 9.71 | 2.05 | 0.09 | 0.17 | 0.01 | 0.01 | 50.45 | 75.68 | -35.5 | -25.8 | -24.1 | |||||
| 420 | 42.35 | 9.54 | 2.06 | 0.11 | 0.19 | 0.02 | 0.01 | 45.72 | 78.03 | -34.5 | -24.7 | -23.4 | -25.3 | -23.8 | |||
| 440 | 49.07 | 8.48 | 1.48 | 0.08 | 0.10 | 0.46 | 40.31 | 82.86 | -32.5 | -24.1 | -23.2 | ||||||
| 460 | 56.82 | 7.06 | 0.83 | 0.05 | 0.03 | 0.45 | 34.77 | 87.70 | -31.3 | -22.5 | -19.5 | ||||||
| 480 | 63.17 | 4.90 | 0.26 | 0.01 | 0.95 | 30.7 | 92.42 | -29.6 | -19.2 | -13.2 | |||||||
| 500 | 69.30 | 2.67 | 0.04 | 0.66 | 27.32 | 96.23 | -28.4 | -14.1 | |||||||||
| 525 | 78.83 | 0.97 | 0.01 | 0.73 | 19.46 | 98.77 | -26.4 | -8.3 | |||||||||
| 550 | 82.11 | 0.32 | 0.02 | 0.98 | 16.57 | 99.59 | -25.8 | -13.0 | |||||||||
| 575 | 85.50 | 0.28 | 1.36 | 12.74 | 0.12 | 99.68 | -24.2 | -14.9 | |||||||||
| 600 | 87.51 | 0.23 | 1.72 | 10.40 | 0.14 | 99.73 | -23.6 | -16.0 | |||||||||
| 625 | 89.27 | 0.14 | 2.01 | 8.58 | 99.84 | -24.1 | -18.9 | ||||||||||
| 650 | -24.0 | -23.7 | |||||||||||||||
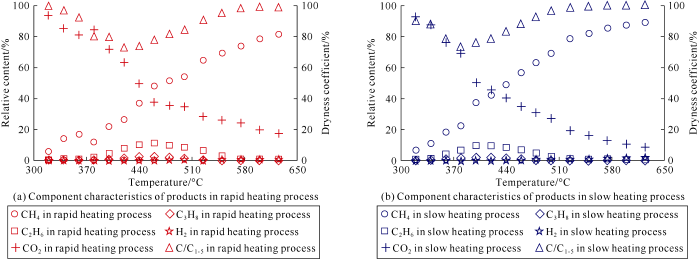
In this study, there was no gas product data at 650 °C, which was caused by gas leakage after isotopic composition analysis. Because the integrity of the data was not affected, and the temperature point (650 °C) data was not supplemented. The relative content of CH4 increased with the increase of temperature in both rapid and slow heating experiments. At the same temperature, the relative content of CH4 in the condition of rapid heating was lower than that of slow heating. In the rapid and slow heating experiments, in which the highest contents of CH4 were 81.64% and 89.72% respectively; and the corresponding temperature was 625 °C. The dryness coefficients (C1/C1-5) of gas products in the two groups of experiments also showed the same change trend, and the temperature of dryness coefficient at the inflection point of the slow heating experiment was lower than that of the rapid heating (Fig. 2).
Fig. 2.
Fig. 2.
The gas components in rapid heating experiment (a) and those of gas components in slow heating experiment (b) of the low mature coal sample from the Ordos Basin, NW China.
The content of heavy hydrocarbon gas increased first and then decreased, which indicates that the heavy hydrocarbon gas was generated and cracked at the same time during the thermal maturation process of coal. In the early stage, generation took dominance, while in the late stage, cracking took dominance. At the point the two processes reached a balance, the relative content of heavy hydrocarbon gas reached the highest. The temperature corresponding to the highest content of heavy hydrocarbon gas was generally 400-500 °C. During the experiments, a small amount of nC4H10, iC4H10, nC5H12, iC5H12 (Table 2) were also produced. As heavy hydrocarbon content first increased and then decreased, the dryness coefficient of the gas product first decreased and then increased. In the early stage, the gas produced in the experiment showed features of dry gas or wet gas (in the slow heating experiment, the gas generated in the early stage showed features of dry gas, while in the rapid heating experiment, the gas showed features of wet gas in the early stage), and the gas produced in the late stage of both experiments showed dry gas characteristics (Fig. 2). In the thermal simulation experiments, a large amount of non-hydrocarbon gases, especially CO2, were formed. And at lower temperatures, the relative content of CO2 took absolute dominance. Under geological conditions, the relative content of CO2 in gas reservoirs was generally low, which might be due to the high solubility of CO2 in water[6]. In addition, CO2 might also participate in diagenesis and enter various diagenetic minerals[30].
2.3. Carbon isotopic compositions of the simulated gases
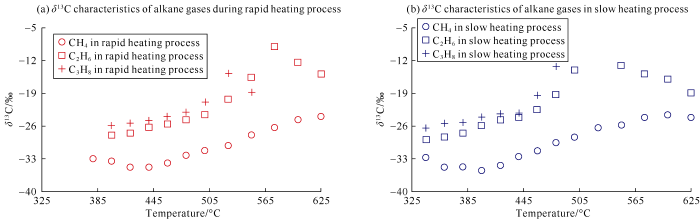
The carbon isotopic composition of natural gas is an important parameter to investigate the geochemical characteristics of natural gas[11,12]. In this work, the carbon isotopic compositions of gas products in the experiments were tested. Because the yields of some gas components at some temperature points were lower than the precision range of the instrument analysis, there were no isotopic values at these temperature points. The detailed carbon isotopic values are shown in Table 2.In the experiments of rapid heating and slow heating, the variations of carbon isotopic composition generally had a similar maturation trend (Table 2). In the rapid heating and slow heating experiments, the δ13C1 values were -34.8‰ to -23.6‰ and -35.5‰ to -24.0‰, δ13C2 values were -28.0‰ to -9.0‰ and -28.9‰ to-8.3‰, δ13C3 values were -25.8‰ to -14.7‰ and -28.9‰ to -8.3‰ respectively. These are similar to the results of previous studies[27, 31-32]. It is worth noting that the inversion of δ13C2 and δ13C3 values in the high temperature stage was found in our experiment (the isotope value became lighter after the maximum value of δ13C2 and δ13C3 appeared in the high temperature stage). This phenomenon was not reported in previous studies. Recently, a few researchers found the inversion of δ13C2 value at high temperature in simulation experiments[22, 26-27]. Generally speaking, the fractionation effect of carbon isotopic composition of alkane gas in the slow heating experiment was stronger than that in the rapid heating experiment.
3. Carbon isotopic fractionation of natural gas in different thermal maturation stages
The hydrocarbon generation model during organic matter thermal maturation has been established through simulation experiments combined with reservoir examples[5]. According to previous studies, there was non-monotonic variation of δ13C1 value in the relatively low maturation stage of organic matter and "anomaly" of carbon isotopic composition of alkane gas at the high to over mature stages. The explanations of these two phenomena are still controversial in academic field. These two phenomena also appeared in our simulation experiments (Fig. 3 and Table 2), so we will focus on the discussion of them below.
Fig. 3.
Fig. 3.
δ13C values of alkane gas in rapid heating experiment (a), and δ13C characteristics of alkane gas in slow heating experiment (b) of the low maturity coal sample from the Ordos Basin.
3.1. Carbon isotopic fractionation of natural gas in relatively low maturity stage
In actual geological background, the rate of temperature rise of source rock is slower than that of any thermal simulation experiment. The isotopic fractionation lag of natural gas formed by thermal maturation of source rock in actual formation should be more obvious than that of thermal simulation experiment product. In our simulation experiments, the massive heavy hydrocarbon gas cracking corresponded to temperatures of about 440-480 ºC. The stage before massive heavy hydrocarbon gas cracking is defined as relatively low mature stage in this paper. In this stage, the yields of CH4 and heavy hydrocarbon gas increased with the increase of source rock maturity. After reaching the cracking temperature of heavy hydrocarbon gas, the yield of heavy hydrocarbon gas began to decrease, but the yield of CH4 increased constantly (Table 1).A large number of previous studies have shown that the value of δ13C1 decreases first and then increases in alkane gas generated by either source rock, kerogen or crude oil at relatively low temperatures[22, 27, 31-32]. In our thermal simulation products, the δ13C1 value also decreased first and then increased. In the rapid temperature rise experiment, δ13C1 reached the minimum value of -34.8‰ at 440 ºC, and then increased with the increase of experiment temperature. In the slow temperature rise experiment, δ13C1 reached the minimum value of -35.5‰ at 400 ºC, and then increased with the increase of temperature (Fig. 3 and Table 2). The inversion of δ13C1 corresponded to the temperatures of 400-440 ºC. In the thermal simulation experiments of marine kerogen and crude oil carried out by Tian et al.[31], the inversion of δ13C1 value at low temperature stage was also found. After the δ13C1 value was higher than the inflection point, the difference of δ13C1 of the same sample at different heating rates gradually decreased[31].Many researchers put forward their own views on the phenomenon that the δ13C1 value first decreased and then increased at the relatively low temperature stage. Shuai Yanhua et al.[32] thought that the inversion of δ13C1 value at relatively low temperature occurred in both closed system and open system thermal simulation experiments, and the structural complexity and heterogeneity of organic matter in reactants were the fundamental reasons of the inversion. Lorant et al.[33] and Hill et al.[34] considered that it was caused by complex precursors in immature or low mature kerogen. Galimov et al.[35] suggested that the organic matter in all the experimental samples had previous transformation history, the residual organic matter from the previous stage was richer in 13C, and the new pyrolysis products were richer in 12C[35]; at the beginning, the residual organic matter contributed a lot to CH4; with the reaction going on, 13C enriched in the new pyrolysis products gradually, resulting in the inversion of δ13C1 value[35]. No matter it is humic organic matter or sapropelic organic matter, in the relatively low mature stage, due to the relatively weak thermal effect, the material generated first must be formed by break of branch chains with relatively lower activation energy and stability. He et al.[29] analyzed the residual elements of lignite after thermal experiment, and found that the organic matter in the low mature stage lost a large amount of oxygen element, so they concluded that the products of early organic matter decomposition were mostly formed by the shedding of functional groups containing heteroatoms like oxygen. In the relatively low mature stage, the functional groups with oxygen and other heteroatoms first fell off, and 12C was likely to enrich in the heteroatom functional groups[29], therefore, CH4 generated at the beginning was richer in 12C, and had lighter δ13C1, and with the thermal maturation going on, the δ13C1 value gradually decreased in the initial stage[29], when the heteroatom functional groups were consumed up, the δ13C1 value gradually became heavier, which was due to the thermal effect of isotopic fractionation[29]. It should be noted that the carbon isotopic inversion of CH4 at low temperature also occurred in the thermal simulation experiments of some pure materials (with no heteroatom functional groups). Tang et al.[36] and Zhang et al.[37] carried out thermal simulation experiments of n-octadecane, and the δ13C1 value in the simulated gas also inversed in the low temperature stage, indicating that the heteroatom functional group was not the necessary factor for the inversion of δ13C1 value in the simulation experiment at low temperature. Tang et al.[36] thought that the isotopic fractionation effect resulted from the activation energy difference between CH4 richer in 12C and that richer in 13C caused the non-monotonic change of δ13C1 value in the low mature stage. Through thermal simulation experiment of n-octadecane, Xiong Yongqiang et al.[38] concluded that some unknown substances with light isotopic composition were formed in the disproportionation reaction during the thermal maturation process, and the decomposition of this new material caused the inversion of δ13C1 value in the initial stage. In the thermal simulation experiment of n-octadecane, the δ13C1 inversion was also found, which indicated that the shedding of heteroatom functional groups was not the essential factor for the early δ13C1 inversion. Both the heterogeneity of organic matter and the isotopic fractionation effect of CH4 richer in 13C and 12C were persuasive to some extent. In essence, the sources of CH4 in the early stage of formation were different.In the relatively low mature stage, the inversion phenomenon might also occur in δ13C2 and δ13C3. However, due to the limited amount of heavy hydrocarbon gas in low maturation stage, it was difficult to accurately detect the carbon isotopic value of heavy hydrocarbon gas. In the relatively low mature stage, the simulated gas products were mainly CH4, CO2 and a small amount of heavy hydrocarbon gas (Table 1, Fig. 1 and Fig. 2). Because of the complexity of CH4 source in the early stage, the carbon isotopic value of CH4 was non-monotonic.
3.2. Carbon isotopic fractionation of natural gas at the high to over mature stages
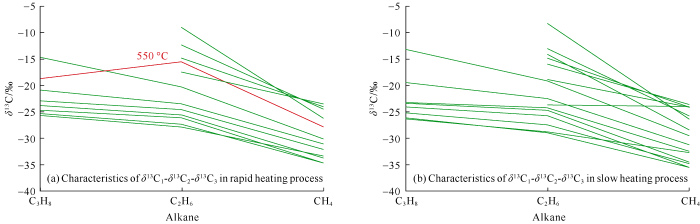
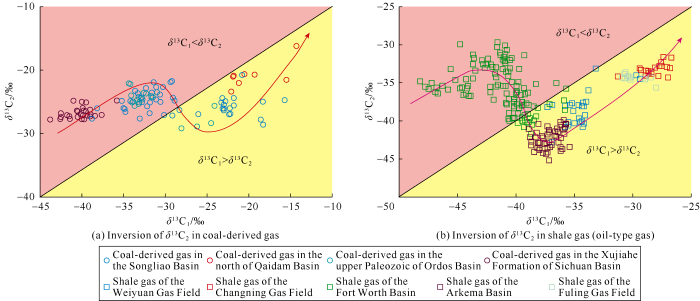
In our gold capsule thermal simulation experiments, there were two kinds of "anomalies" of carbon isotopic composition of alkane gas. One was the non-monotonic change of δ13C1 value in the initial stage, the other was the inversion of carbon isotope value of heavy hydrocarbon gas at the high to over mature stages and the partial reversal of carbon isotopic series at a temperature point (550 ºC in rapid heating).Alkane gas from natural maturation of organic matter generally has positive carbon isotopic series (with the increase of carbon number, the carbon isotopic value of alkane gas increases gradually)[12, 17]. However, in recent years, many cases of "abnormal" alkane gas isotopic composition were found in many gas reservoirs, such as partial reversal of isotopic series[13,14], negative carbon isotopic series[17], and the inversion of alkane gas isotopic composition[13,14]. These "anomalies" of isotopic composition often occur at the high to over mature stages, which reflects the complex formation mechanisms of natural gas at the high to over mature stages.In our thermal simulation experiments, the carbon isotopic composition of alkane gases generally exhibited positive series, but when the temperature rose rapidly to 550 ºC, the carbon isotopic series of alkane gas was partially reversed (Figs. 3a and 4a). By reviewing the results of previous thermal simulation experiments, it could be found that the composition and yield characteristics of alkane gas were more deeply studied before[39], but the formation mechanisms and isotopic values of natural gas at the high to over mature stages were relatively less studied. Most researches on the isotopic composition of alkane gas before were limited to δ13C1[36], and the research on heavy hydrocarbon was relatively weak[40]. Some researchers studied the carbon isotopic composition of alkane gas, but the simulation experiments were at low temperatures (the maturation degree of organic matter was not high enough), and the alkane gas in most cases showed positive carbon isotopic composition series[41]. A few researchers found negative carbon isotopic series or partial carbon isotopic composition reversal through thermal simulation experiments. For example, Du et al.[21] carried out thermal simulation experiment on lignite in closed system at ultra-high pressure of 1-3 GPa and temperatures of 500-700 ºC, and found negative and partial reversal of carbon isotopic composition in alkane gas. However, the pressure of organic matter thermal maturation system was generally 20-60 MPa, so the pyrolysis in ultra-high pressure system couldn’t represent the actual process of thermal maturation of source rock. Zhang et al.[23] carried out thermal simulation experiments with hydrogen and inorganic materials of three different carbon sources to simulate the Fischer-Tropsch synthesis reaction, and positive carbon isotope series, partial reversal carbon isotopic series and negative carbon isotopic series were found in the simulation products. However the actual thermal maturation of source rocks was mainly the conversion of complex organic macromolecules to small molecules, so the Fischer-Tropsch synthesis experiment might be quite different from the thermal maturation of organic matter in geological bodies. The simulation experiments designed by Du et al.[21] and Zhang et al.[23] couldn’t well represent the formation mechanism of alkane gas in actual gas reservoirs.In our thermal simulation experiment, partial carbon isotopic reversal only occurred at one temperature point (red line in Fig. 4a), but the carbon isotopic inversion of C2H6 and C3H8 occurred in the high temperature stage (Fig. 3). In recent years, Mao Rong et al.[22], He et al.[26], and Gao et al.[27] observed the inversion of δ13C2 value in the high temperature stage of pyrolysis experiments, but this paper was the first reporting δ13C3 inversion at high temperature phase in thermal simulation experiment. It is worth noting that the reversal of isotopic series, negative isotopic series and carbon isotopic inversion of heavy hydrocarbon gas (C2H6 and C3H8) have been confirmed by researchers in several gas reservoirs[12,13,14,15,16,17]. Based on previous studies[17, 42-48], we made the correlation diagrams of δ13C1 and δ13C2 values of coal-derived gas (Fig. 5a) and shale gas (oil-type gas) (Fig. 5b). It was found that the inversion and reversal of isotopic composition would occur in both coal-derived gas and oil-type gas at certain thermal maturation degree. Through the analysis of our experimental products, it is inferred that the inversion and reversal of carbon isotope may be a common phenomenon when organic matter reaches certain thermal maturation stage. Only the simulation experiment can dynamically study the formation process of natural gas, so this phenomenon can be well reflected in the experiment.
Fig. 4.
Fig. 4.
The δ13C1-δ13C2-δ13C3 values from rapid temperature rise (a) and δ13C1-δ13C2-δ13C3 from slow temperature rise (b) of low mature coal sample from the Lower Permian Shanxi Formation in the eastern Ordos Basin.
Fig. 5.
Fig. 5.
Carbon isotopic inversion of ethane in coal-derived gas (a) and ethane carbon isotopic inversion in shale gas (oil-type gas) (b).
Before the discovery of shale gas with abnormal isotopic composition, there were four common views on the genetic interpretation of the abnormal isotopic series of natural gas[49]: (a) mixing of organic gases from different sources or of different maturity from the same source; (b) mixing of organic gas and inorganic gas; (c) migration and fractionation of natural gas; and (d) microbial action. In recent years, with the discovery of a large number of cases of isotopic abnormal shale gas, many researchers have tried to find the causes of the inversion and reversal of isotopic series in shale gas. There are two types of views: (a) in over mature stage, water reacts with CH4 to produce CO2 with lighter carbon isotopic composition and H2, and CO2 and H2 react again to produce heavy hydrocarbon gas with light carbon isotopic composition[14, 19], resulting in the inversion and reversal of isotopic composition[14, 19]; and (b) the mixing of kerogen cracking gas and oil cracking gas results in these abnormalities[15].The generated CO2 and H2 reacted again to form heavy hydrocarbon gas with light carbon isotopic composition, thus forming the abnormal isotopic composition. The reaction of CO2 and H2 at high temperature belonged to Fischer-Tropsch synthesis. We thought this was only a theoretical model or hypothesis. Considering the reactants (CO2 and H2) needed for Fischer-Tropsch reaction[50,51], the reactants didn’t need to be formed by the oxidation-reduction reaction of CH4. Both our thermal simulation experiment and previous studies showed that CO2 had been formed in the maturation process of source rocks, and H2 was also generated during the graphitization of organic matter. The question was whether this reaction could occur in the formation system. Generally, the Fischer-Tropsch reaction in the laboratory took place under the condition of transition metal as catalyst, and the reaction temperature of Fischer- Tropsch synthesis was not very high (200-400 ºC)[50,51]. Compared with the laboratory conditions, there were less transition metals in the formation[52]. Whether a very small amount of transition metals could make the Fischer-Tropsch synthesis reaction take place under the formation conditions needed more evidence to support. The main function of catalyst was to reduce the activation energy of the reaction. If there was not enough transition metal in the formation, the Fischer-Tropsch synthesis at relatively low temperature couldn’t occur smoothly. Although CO2 could be generated in the whole maturation process of source rocks, the formation of H2 mainly occurred in the graphitization process, i.e., high to over mature stages. Whether there were enough transition metals in source rocks to catalyze the Fischer-Tropsch synthesis also needed more evidences. Therefore, we thought that it was not convincing to explain the inversion or reversal of carbon isotopic composition by using the viewpoint that CO2 and H2 reacted to produce heavy hydrocarbon gas with light carbon isotope. In our thermal simulation experiments, there was no microbial, no water, no gas migration. And the simulated gas was typical organic gas generated by coal. The thermal simulation experiment of source rock in closed system showed a continuous accumulation of natural gas, and there was indeed a mixture of natural gas formed by thermal maturation of organic matter in different periods. In the high temperature stage, the heavy hydrocarbon gas had begun to crack gradually, and the secondary cracking of the retained hydrocarbon (mainly referred to chloroform asphalt "A") in the source rock should be earlier than that of the heavy hydrocarbon gas. Therefore, it might be improper to think that the secondary cracking of the retained hydrocarbon was the cause of "abnormal" geochemical characteristics of natural gas at the high to over mature stages.In our experiment, the inversion and partial reversal of carbon isotopic series occurred in the higher temperature stage. It is worth noting that the "abnormal" phenomena such as inversion and reversal of carbon isotope composition in actual gas reservoirs generally occurred at the high to over mature stages[15,16,17], which indicated that natural gas at the high to over mature stages might be the key factor causing the "abnormal" geochemical characteristics, which also demonstrated the complexity of the formation mechanism of high-over mature natural gas. In our study, it was believed that the inversion of carbon isotopic value of heavy hydrocarbon gas at the high to over mature stages (the carbon isotopic value became lighter) might indicate that the source of heavy hydrocarbon gas had changed at the high to over mature stages, i.e., there was a new material source of heavy hydrocarbon gas at the high to over mature stages. This phenomenon might be similar to the inversion of δ13C1 in the low mature stage. After the carbon isotopic composition of heavy hydrocarbon gas reached the maximum value, the carbon isotopic value of heavy hydrocarbon gas inversed and became lighter gradually (Fig. 3). To further analyze this phenomenon, it is necessary to analyze the thermal maturation structure of organic matter. With the thermal maturation of source rock, both sapropelic and humic organic matter would continuously enrich carbon and dehydrogenate, and aromatization or graphitization[25, 39]. Therefore, the study on the structure of organic matter in high mature stage would help us understand the mechanisms of natural gas formation and isotopic fractionation. Mi et al.[39] carried out nuclear magnetic resonance analysis on coal samples of different maturities. The structure analysis of coal samples showed that when Ro value was greater than 3.0%, coal mainly contained a large number of aromatic rings, and the side chains were mainly methyl[39]. Therefore, at the high to over mature stages (especially the over mature stage), and demethylation of ring structures and aromatic nuclei should be the main way of gas generation of the source rock[25, 38, 53]. It should be a good point to investigate the formation and isotopic fractionation mechanisms of natural gas at the high to over mature stages from the thermal simulation experiment of aromatic hydrocarbon. As humic organic matter contained more aromatic core structure, it may contribute more gas by aromatic hydrocarbon demethylation than sapropelic organic matter[25, 38, 53]. No matter it is sapropelic or humic organic matter, at the high to over mature stages, the gas generated by dehydrogenation of methyl side chains should be CH4. However, the actual results of exploration and thermal simulation have confirmed that the gas from the high to over mature stages of thermal simulation experiment (regardless of shale gas or coal-derived gas) contains a certain amount of heavy hydrocarbon gas[46,47,48]. The heavy hydrocarbon gas may contain a part not fully cracked formed by the thermal maturation of organic matter in the early stage, but as the carbon isotopic composition of heavy hydrocarbon gas reverses, there should be lighter carbon isotope heavy hydrocarbon gas formed. Although obvious partial reversal of carbon isotopic series of alkane gas only occurred at one temperature point in our thermal simulation experiment (Fig. 4a), the reversal of carbon isotopic series at the high to over mature stages has been found in a few natural gas reservoirs[48]. Fusetti et al.[54] found that a large amount of CH4 could be formed during the pyrolysis of 1,2,4-trimethylbenzene in the gold capsule simulation system. Gas generation by organic matter at the high to over mature stages may be mainly side chain shedding of aromatics. Unfortunately, Fusetti et al.[55] did not conduct in-depth analysis on carbon isotopic composition of alkane gas.Aromatic hydrocarbon is one of the main products of organic matter at the high to over mature stages. In order to further explore the formation mechanisms of natural gas at the high to over mature stages, we took toluene as an example to discuss the characteristics of aromatic hydrocarbon cracking products, and then compared the geochemical characteristics of the products at the high to over mature stages. Toluene thermal simulation experiment was carried out at different temperature conditions. The carbon isotopic values of alkane gas in the experimental products are shown in Table 3.
Table 3 Carbon isotopic composition of alkane gas in toluene thermal simulation.
| Temperature/ºC | δ13C/‰ | ||
|---|---|---|---|
| CH4 | C2H6 | C3H8 | |
| 450 | -27.7 | -28.1 | -23.5 |
| 475 | -31.8 | -31.0 | -23.1 |
| 500 | -30.5 | -28.8 | -19.3 |
| 525 | -29.9 | -24.4 | -15.1 |
| 550 | -29.5 | -20.4 | -16.9 |
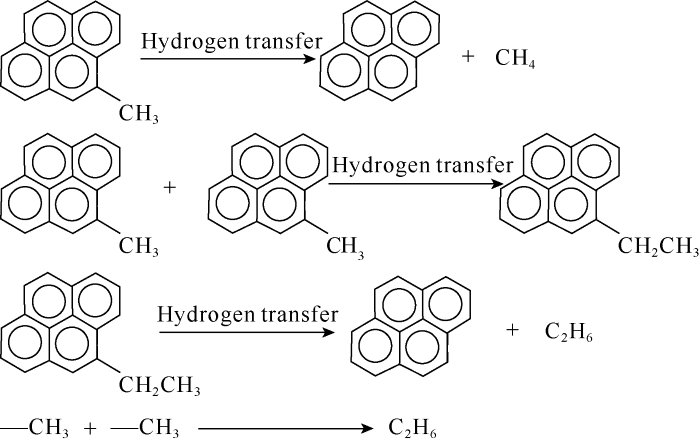
The toluene thermal simulation in gold capsule was carried out. Due to the high stability of aromatic compounds, the temperature was rapidly raised to the target temperature at 20 ºC/h, and then kept constant for 72 h. It was found that not only CH4 but also a small amount of heavy hydrocarbon gas were formed in the thermal simulation experiment of toluene, and partial reversal of carbon isotopic series of alkane gas occurred (Table 3). The initial temperature of this experiment was 325 ºC, but when the simulated temperature was lower than 450 ºC, there was little gas produced, ie., toluene hardly crack into gas before 450 ºC, and gas products were detected after 450 ºC. Combined with the structural characteristics of coal at the high to over mature stages and the toluene pyrolysis experiment, it could be concluded that the alkane gas formed at the high to over mature stages of organic matter might mainly come from the desorption of aromatic compounds (Fig. 6). It was considered that at the high to over mature stages, on the one hand, the dehydrogenation of the detached methyl could form CH4; on the other hand, the linkage between methyl and methyl could form a small amount of C2H6; in addition, the polymerization of aromatic compounds could form ethyl, and ethyl could also form a small amount of ethane through hydrogen transfer (hydrogenation) (Fig. 6). At the high to over mature stages, CH4 was mainly formed by demethylation of alkane gas. In the experiment of toluene pyrolysis at 550 ºC, it could be found that the C3H8 also showed carbon isotopic value inversion, and the partial inversion of carbon isotopic series did appear in the pyrolysis products of toluene (450 ºC, Table 3). With the increase of temperature, the carbon isotopic composition of toluene pyrolysis products also showed obvious fractionation effect. It was believed that the formation of CH4 was due to the demethylation of aromatic rings in kerogen, and the heavy hydrocarbon gas mainly came from the polymerization of methyl (Fig. 6). Due to the stability of aromatic compounds, the short side chains of aromatic structures in kerogen fell off later[38, 53].
Fig. 6.
Fig. 6.
Schematic diagram of aromatic compounds shedding side chains to generate CH4 and C2H6.
The isotopic fractionation effect of aromatic compounds lags behind that of kerogen cracking gas and retained hydrocarbon cracking gas. In the high temperature stage, the formation and cracking of heavy hydrocarbon gas were in a dynamic state. There were heavy hydrocarbon gases cracked at high temperature, and a very small amount of heavy hydrocarbon gas formed by the aromatics in kerogen under high temperature. The carbon isotopic fractionation effect of the early formed natural gas was inconsistent with that of the later formed natural gas, i.e., the carbon isotopic fractionation of the late formed natural gas was lagging behind.
When the organic matter evolved into its structure, which was mainly aryl methyl side chain, the carbon isotopic composition of the detached methyl carbon was usually heavier than that of the relatively low mature stage. However, this didn’t mean that all the methyl groups removed from the coal structure at this time were -13CH3 and also contained a large amount of -12CH3[36], but there were more -13CH3 in the methyl groups removed from the coal structure at lower maturity. That was to say, both -12CH3 and -13CH3 were separated from the parent structure. In the process of forming C2H6 and C3H8 (small amount) from coal structure, because -12CH3 was lighter than -13CH3, its energy and chemical activity were stronger, so the probability of -12CH3 connecting with -12CH3 was greater than that of -12CH3 connecting -13CH3 and-13CH3 connecting -13CH3, which might lead to the enrichment of more 12C in C2H6 and C3H8 generated by methyl linkage. Therefore, δ13C2 value might be lighter than δ13C1 (Table 3), and δ13C3 value might be lighter than δ13C2 (Table 2). A small amount of C2H6 and C3H8 formed in the over mature stage might cause abnormal isotopic composition, such as inversion of carbon isotopic composition (Table 2). If the isotopic composition is inverted to a certain extent, causing the δ13C2 value higher than the δ13C3 (Fig. 4a), then the partial reversal of carbon isotope composition of C2H6 and C3H8 was formed. If δ13C1 value was higher than δ13C2, then a partial reversal of carbon isotopic compositions of CH4 and C2H6 was formed (Table 3). Therefore, the aromatic demethylation of organic matter and the carbon isotopic fractionation during the formation of heavy hydrocarbon gas by methyl linkage might be an important reason for the inversion or reversal of carbon isotopic composition of natural gas (including shale gas) at the high to over mature stages.
Organic matter thermal maturation and gas generation were very complex processes, and the source of natural gas was complex[5,32,35,38,53]. The carbon isotopic compositions of natural gas formed by different materials in different periods had fractionation effect. Especially, the organic matter rich in aromatic structure in the late stage could form alkane gas with light carbon isotopic composition. Because the content of heavy hydrocarbon gas was low, even if the amount of heavy hydrocarbon gas formed in the late stage was very little, it might also have an important impact on the δ13C of heavy hydrocarbon gas. The fractionation effect of δ13C1, δ13C2 and δ13C3 were different, which had an important relationship with the amount of alkane gas. The more the amount of alkane formed in the early stage, the more difficult it was for the later formed alkane gas to affect its isotopic composition. Therefore, the fractionation effect of δ13C1 was slower than that of δ13C2, and the fractionation effect of δ13C2 was slower than that of δ13C3. On the one hand, the lighter δ13C alkane gas formed in the late stage could form the δ13C inversion phenomenon of heavy hydrocarbon gas; on the other hand, the reversal phenomenon of δ13C series of alkane gas could be formed by aromatic structure dealkylation side chain.
4. Enlightenment of thermal simulation on isotopic composition series anomalies of natural gas under geological conditions
Since natural gas geology and geochemistry became an independent discipline, the analysis of the characteristics of isotopic composition has become one of the most useful means to carry out natural gas research[5,6]. In previous studies, it was generally believed that organic alkane gas had positive carbon isotopic series, while inorganic alkane gas had negative carbon isotopic series[12]. With the deepening understanding of natural gas geology and geochemistry, the partial reversal and even negative carbon isotopic series of alkane gas were generally discovered in petroliferous basin all over the world[16,17]. The negative carbon isotopic series of natural gas can also be organic origin, which is widely accepted now. However, the interpretation of inversion or anomaly of carbon isotopic series of alkane gas was mostly based on theoretical speculation[17]. Thermal simulation experiment could explore the internal relationship of gas geochemical characteristics from the perspective of hydrocarbon formation, which was conducive to the study from the perspective of mechanism[28]. Through the thermal simulation experiment of low mature coal and toluene, we tried to investigate the origin of "anomaly" of isotopic series from the formation mechanism of alkane gas. There were a series of abnormal carbon isotopic compositions of alkanes in both coal-derived gas and oil-type gas (Fig. 5), which were mainly manifested at the high to over mature stages. The main gas production of organic matter at the high to over mature stages was aromatic structure shedding side chain or methylation. We speculated that demethylation or side chain shedding of aromatic structure was an important reason for the formation of "anomaly" of carbon isotopic composition series of alkane gas at the high to over mature stages. This conjecture was not a complete negation of previous views, but an analysis from a new perspective. Li et al.[56] found that there were four stages of δ13C1 fractionation in shale gas production process, and proposed that the carbon isotopic fractionation of shale gas was mainly controlled by adsorption, desorption and diffusion, and the δ13C1 value in each stage was mainly affected by the enrichment of 13C and 12C methane fractionation. Their viewpoint was further demonstrated by kinetic calculation. The viewpoint proposed by Li et al.[56] was consistent with that obtained by our thermal simulation experiment. The gas formed in thermal simulation experiment only experienced hydrocarbon generation, and did not experience the accumulation process such as geological gas reservoir. Moreover, the heating rate of organic matter in the actual geological condition was extremely slow. The existing experimental technical conditions can not completely simulate the actual geological conditions, so the thermal simulation experiment has certain limitations. However, the limitations don’t affect the thermal simulation experiment to be one of the most effective methods to analyze hydrocarbon gas formation under present conditions.
5. Conclusions
The thermal simulation experiment of a low mature coal sample from the Lower Permian Shanxi Formation in the eastern Ordos Basin was carried out in a gold capsule closed pyrolysis system at slow and rapid heating rates. The maximum yields of alkane gas were 302.74 ml/g and 230.16 ml/g, respectively. In the slow heating process, the organic matter reaction was more sufficient and the δ13C fractionation effect more obvious. In the simulation experiments at slow and rapid heating rates, δ13C1 values were -34.8‰ to -23.6‰ and -35.5‰ to -24.0‰, δ13C2 values were -28.0‰ to -9.0‰ and -28.9‰ to -8.3‰, and δ13C3 values were -25.8‰ to -14.7 ‰ and -26.4‰ to -13.2‰, respectively.
In both heating processes, δ13C1 turned lighter first and then heavier. The non-monotonic change of δ13C1 was caused by different sources of CH4 formed in the early stage, and the cause of different sources of CH4 was the heterogeneity of organic matter or the difference of activation energy between CH4 rich in 12C and that rich in 13C.
The inversion of δ13C in heavy hydrocarbon gas can occur in highly mature shale gas (oil-type gas) or in coal-derived gas. The thermal simulation experiment further proved that heavy hydrocarbon gas can have inversion of δ13C at the high to over mature stages. The inversion of δ13C indicates that the sources of heavy hydrocarbon gas at the high to over mature stages are complex, and heavy hydrocarbon gases from different sources show fractionation effect in different times. Based on the thermal simulation experiment of toluene, it is concluded that the δ13C fractionation effect caused by demethylation of aromatic structure and methyl linkage may be an important reason for δ13C inversion and reversal of alkane gas at the high to over mature stages.
Reference
Some problems in the study of petroleum geology
Time-limit and yield of natural gas generation from different origins and their effects on forecast of deep oil and gas resource
Discussion on the upper maturity limit and gas potential limit of marine kerogen: A case study of the Tarim Basin
The hydrogen and carbon isotopic composition of methane from natural gases of various origins
DOI:10.1016/0016-7037(80)90155-6 URL [Cited within: 1]
Classification and thermal history of petroleum based on light hydrocarbons
DOI:10.1016/0016-7037(83)90143-6 URL [Cited within: 1]
Genetic characterization of natural gas
Origin of nitrogen-rich natural gases in the California Great Valley: Evidence from helium, carbon and nitrogen isotope ratios
DOI:10.1016/0016-7037(88)90356-0 URL [Cited within: 1]
Research progress of gas geochemistry during the past decade in China
Carbon and hydrogen isotopes of methane, ethane, and propane: A review of genetic identification of natural gas
DOI:10.1016/j.earscirev.2018.11.017 URL [Cited within: 5]
Gas isotope reversals in fractured gas reservoirs of the western Canadian Foothills: Mature shale gases in disguise
Isotopically reversed gases (delta(13)C methane > delta(13)C ethane > delta(13)C propane) occur in fractured mixed clastic-carbonate reservoirs of the Permian and the Triassic in the foothills at the western edge of the Western Canada sedimentary basin (WCSB). The delta(13)C methane values (-42 to 24 parts per thousand), gas dryness, and organic maturity (R(0) > 2.2) are indicative of mature gases, and gas maturity generally increases with reservoir age and from the southeast to the northwest. The delta(13)C ethane values range from -44 to -25, with the less negative values in isotopically normal gases to the northeast of the gas fields we studied. To explain the gas isotope reversals observed in the WCSB foothills, we adopt the concept of a closed-system shale, in which simultaneous cooking of kerogen, oil, and gas yields gas with light delta(13)C ethane and heavy delta(13)C methane. This gas was released from shales and trapped in fractured folds of brittle clastic-carbonate rocks during deformation and thrust faulting of the Laramide orogeny, creating some of the most prolific gas pools. These gases are actually mature shale gases. Local high abundances of H(2)S and CO(2) are most likely the products of thermochemical sulfate reduction (TSR) reactions in anhydrite-rich interbeds and underbeds that admixed to the released shale gas during the tectonic event. No evidence exists that TSR is responsible for the isotope reversals. Variations in delta(13)C ethane are likely caused by local differences in thermal history, the timing of gas release from shale, and the timing of the fault and fold development. Less negative delta(13)C ethane values (resulting in isotopically normal gases) to the northeast of the fields and in
Isotopic reversal (‘rollover’) in shale gases produced from the Mississippian Barnett and Fayetteville formations
DOI:10.1016/j.marpetgeo.2011.06.009
URL
[Cited within: 6]

Ethane, propane, and carbon dioxide show reversed carbon isotopic maturity trends in natural gas produced from the Barnett and Fayetteville Shales at thermal maturities greater than similar to 1.5% VRE. At this high level of thermal maturity, the iso-butane to n-butane ratio also reverses, suggesting wet gas cracking has occurred, generating more gas molecules in the same volume resulting in overpressure, and increased stabilized production rates in the Barnett. Hydrothermal fluids from the nearby Ouachita Thrust front apparently enhanced the maturity of the Fayetteville and Barnett shales. Water-hydrocarbon reactions at these high maturities may account for the isotopic reversals. (C) 2011 Elsevier Ltd.
Isotopic reversals with respect to maturity trends due to mixing of primary and secondary products in source rocks
DOI:10.1016/j.chemgeo.2012.07.025 URL [Cited within: 7]
Isotope reversals and universal stages and trends of gas maturation in sealed, self-contained petroleum systems
DOI:10.1016/j.chemgeo.2012.08.002
URL
[Cited within: 5]

Isotope geochemistry is now a tool for shale gas exploration, largely due to the association of isotope reversals with mature, highly productive shale gas. Its utility, however, depends on an understanding of the isotope systematics for the particular region of interest, as well as for shale gas maturation in general. This paper reviews and re-examines isotope data from four published papers that include shale gas from the Barnett and Fayetteville Shales (Rodrigez and Philp, 2010; Zumberge et al., 2012), and gas from fractured reservoirs in the Appalachians (Burruss and Laughrey, 2010) and the Foothills of the Western Canada Sedimentary Basin (WCSB) (Tilley et al., 2011). New shale and tight sandstone gas data are also presented for the WCSB. Comparisons of these data show that the progression through three stages of gas maturation (pre-rollover zone, rollover zone and post-rollover zone) is universal in sealed, self-contained petroleum systems and that each zone has characteristic isotopic relationships and trends that are seen in all areas examined.
Gases in the pre-rollover zone are isotopically normal (delta C-13(methane)
Secondary origin of negative carbon isotopic series in natural gas
DOI:10.1016/j.jnggs.2016.02.002 URL [Cited within: 9]
Demethylation as a mechanism for isotopic reversals of shale gas generated at over maturity
DOI:10.1016/j.jaap.2018.08.015 URL [Cited within: 1]
Carbon and hydrogen isotopic reversals in deep basin gas: Evidence for limits to the stability of hydrocarbons
DOI:10.1016/j.orggeochem.2010.09.008
URL
[Cited within: 4]

Abstract
During studies of unconventional natural gas reservoirs of Silurian and Ordovician age in the northern Appalachian basin we observed complete reversal of the normal trend of carbon isotopic composition, such that δ13C methane (C1) >δ13C ethane (C2) >δ13C propane (C3). In addition, we have observed isotopic reversals in the δ2H in the deepest samples. Isotopic reversals cannot be explained by current models of hydrocarbon gas generation. Previous observations of partial isotopic reversals have been explained by mixing between gases from different sources and thermal maturities. We have constructed a model which, in addition to mixing, requires Rayleigh fractionation of C2 and C3 to cause enrichment in 13C and create reversals. In the deepest samples, the normal trend of increasing enrichment of 13C and 2H in methane with increasing depth reverses and 2H becomes depleted as 13C becomes enriched. We propose that the reactions that drive Rayleigh fractionation of C2 and C3 involve redox reactions with transition metals and water at late stages of catagenesis at temperatures on the order of 250–300 °C. Published ab initio calculated fractionation factors for C–C bond breaking in ethane at these temperatures are consistent with our observations. The reversed trend in δ2H in methane appears to be caused by isotopic exchange with formation water at the same temperatures. Our interpretation that Rayleigh fractionation during redox reactions is causing isotopic reversals has important implications for natural gas resources in deeply buried sedimentary basins.
Reaction kinetics of stable carbon isotope in natural gas-insights from dry, open system pyrolysis experiments
Stable carbon isotope compositions of gaseous hydrocarbons produced from high pressure and high temperature pyrolysis of lignite
DOI:10.1016/S0146-6380(02)00158-4 URL [Cited within: 3]
Study on the hydrocarbon generation characteristics of different coaly source rocks by gold-tube pyrolysis experiments
From the simulation experiments on the hydrocarbon generation of Jurassic coal,coaly mudstone and carbon mudstone from Ordos basin,it is indicated that the oil generation characteristics of three coaly source rocks are distinct from each other in pyrolysis.The hydrocarbon generation potential of the three coal bearing source rocks were all relatively low compared to a typical lacustrine or marine source rock.Coaly mudstone had a highest amounts of oil yields with a maximum of about 20mg/gTOC,which is twice as that of coal and carbon mudstone.Hence,coal bearing source rocks seem impossible to form extensive oil reservoir.The maximum gas yields of coal,coaly mudstone and carbon mudstone is 184-212mL/gTOC.Wherein,coal had a highest amounts of gas yields in the three source rocks,the largest amount of that generated from coaly mudstone was less than 3m3/trock.The isotope distributions of hydrocarbon gases derived from coal and carbon mudstone were similar.The carbon isotope of methane from coaly mudstone is 3‰-5‰ lighter than that from coal at the same temperature.The results of simulation experiments on the gas yields from the three source rocks demonstrated that coal should be the dominant contributor for the accumulation of coal genetic gas.
Synthesis of hydrocarbon gases from four different carbon sources and hydrogen gas using a gold-tube system by Fischer-Tropsch method
DOI:10.1016/j.chemgeo.2013.03.016 URL [Cited within: 4]
Experimental investigations about the effect of pressure on gas generation from coal
DOI:10.1016/j.orggeochem.2014.05.012
URL
[Cited within: 2]

Closed-system pyrolysis experiments were conducted on a coal sample with a maturity of 0.57 %R-o using gold tubes pressured to 10 MPa, 25 MPa, 50 MPa, 75 MPa and 100 MPa to investigate the influence of increasing pressure on gas generation. The variation of gaseous components generated by coal, the H/C atomic ratio and the vitrinite reflectance of pyrolysis residues with pressure and temperature indicate that pressure does not linearly impact gas generation from coal. Pressure has no effect on primary gas generation, but it does affect secondary gas generation. Retardation of secondary generation is highest at 50 MPa. Our investigations reveal that previously published and partly opposing results about the effect of pressure on hydrocarbon generation can be mainly explained by the difference in the pressure range under which those experiments were conducted. Our experimental results demonstrate that hydrocarbon generation is generally least retarded at pressures exceeding 75 MPa. Nevertheless, hydrocarbon generation in natural systems usually occurs at pressures of 10-60 MPa (1-6 km burial depth) and might therefore be secondarily controlled by pressure retardation effects. (C) 2014 Elsevier Ltd.
The upper thermal maturity limit of primary gas generated from marine organic matters
DOI:10.1016/j.marpetgeo.2017.06.045 URL [Cited within: 5]
Pyrolysis involving n-hexadecane, water and minerals: Insight into the mechanisms and isotope fractionation for water-hydrocarbon reaction
DOI:10.1016/j.jaap.2018.01.009 URL [Cited within: 4]
Gas generation and its isotope composition during coal pyrolysis: The catalytic effect of nickel and magnetite
DOI:10.1016/j.fuel.2018.02.118 URL [Cited within: 6]
Research status on thermal simulation experiment and several issues of concern
DOI:10.1016/j.jnggs.2018.11.006 URL [Cited within: 3]
The evolution of chemical groups and isotopic fractionation at different maturation stages during lignite pyrolysis
DOI:10.1016/j.fuel.2017.09.085 URL [Cited within: 4]
Effect and quantitative evaluation of CO2 derived from organic matter in coal on the formation of tight sandstone reservoirs
DOI:10.1007/s11430-012-4565-2 URL [Cited within: 1]
Comparison of gas generation and carbon isotope fraction of methane from marine kerogen and crude oil-cracking gaes
DOI:10.1016/0016-7037(72)90121-4 URL [Cited within: 4]
Kinetic model for the stable carbon isotope of methane: The state of the art
Carbon isotopic and molecular constraints on the formation and the expulsion of thermogenic hydrocarbon gases
DOI:10.1016/S0009-2541(98)00017-5 URL [Cited within: 1]
Insight into cracking based on laboratory experiments
DOI:10.1016/S0146-6380(03)00173-6 URL [Cited within: 1]
Isotope organic geochemistry
DOI:10.1016/j.orggeochem.2006.04.009 URL [Cited within: 4]
Mathematical modeling of stable carbon isotope ratios in natural gases
DOI:10.1016/S0016-7037(00)00377-X URL [Cited within: 4]
Pyrolysis kinetics of pure n-C18H38 (I): Gaseous hydrocarbon and carbon isotope evolution
N-octadecane pyrolysis and its geochemical significance
DOI:10.1007/BF02890456 URL [Cited within: 5]
Upper thermal maturity limit for gas generation from humic coal
DOI:10.1016/j.coal.2015.08.009 URL [Cited within: 4]
Expirical carbon isotope maturity relationships for gases from algal kerogens and terrigenous organic matter, based on dry, open-system pyrolysis
DOI:10.1016/S0146-6380(96)00090-3 URL [Cited within: 1]
Stable carbon isotope compositions of gaseous hydrocarbons in pyrolysis experiment and geochemical significance
In this paper,different types of samples,including crude oil,chloroform bitumen A,oil fractions (saturated hydrocarbon,aromatic hydrocarbon and asphaltene),source rock and kerogen,were performed in pyrolysis experiment at high temperature.Carbon isotope composition of gaseous hydrocarbons was measured.With increase of pyrolysis temperatures,δ13C values of gaseous hydrocarbons from different types of samples (i.e crude oil,chloroform bitumen A,kerogen,source rock,and saturated hydrocarbon) were various,with heavy value of 13C at low temperature,lighter value at moderate temperature,and heavier value at higher pyrolysis temperature.A normal serial of stable carbon isotopes for methane,ethane and propane occurred before pyrolysis temperature less than 700℃.But,reverse trend happened at pyrolysis temperature more than 750℃.The different change of stable carbon isotope values and normal serial for gaseous hydrocarbons from aromatic hydrocarbon and asphaltene existed.Δδ13C2-1 values for gaseous hydrocarbon from all samples increased with pyrolysis temperature increasing.Values of Δδ13C3-1and Δδ13C3-2 were various for samples.A position correlation between δ13C2—δ13C3 and Ln(C2/C3)occurred with pyrolysis temperature increasing.
Nayural gas genetic type and accumulation characteristics in Erboliang III Structure in north margin of Qaidam Basin
A study on geochemical character and origin of natural gas in Dehui Fault Depression of the southern Songliao Basin
Dehui fault depression has been explored for more than forty years, while less b reakthrough has been made since 1990s,so it is significantly to deep understand the geochemical character and origin of deep natural gas to the next exploration .Studying on its components and carbon isotopes , with the research on geologic al condition, show that: carbon dioxide is organic origin, shallow dry gases in Nongan gas-field are formed correlating with fractionation duing vertical migra tion and overpressure in Quan No.3 interval in mudstone besides parent materials , but dry gases from the Yingcheng and Shahezi formations origin from high matur e source rock, gases can be subdivided into petroliferous gases, coal derived ga ses, hybrid gases and biogenic gases according to the types of parent material a nd the second one is the major part. It is also believed that part normal mature gas which has not been destroyed and abundant high mature gas accumulate in the Yingcheng and Shahezi formations ,and large-scale gas reservoir may be formed in these formations for their enough hydrocarbon source.
A gas source rocks and gas genetic type in Shuangcheng-Taipingchuan area of Songliao Basin
Genetic types of natural gas and its exploration direction in Lishu Fault Sag, Songliao Basin
The hydrogen isotopic characteristics of the Upper Paleozoic natural gas in Ordos Basin
DOI:10.1016/j.orggeochem.2014.01.020
URL
[Cited within: 1]

Upper Paleozoic natural gas in the Ordos Basin is typical coal-associated gas. Natural gases in Sulige, Yulin and Zizhou gas fields were systematically analyzed in this study. Based on the hydrogen isotopes of CH4, C2H6 and C3H8 components in natural gases, and combined with the natural gas compositions and carbon isotopes and fluid inclusions homogenization temperature measurement of reservoir rocks, we have discussed the source rock depositional environment, gas origin and maturity, and gas migration and accumulation. The results show that methane hydrogen isotope of natural gas not only can indicate the type of precursor organic matter and depositional redox condition, but also is closely associated with the maturity of natural gas. The difference between delta D-C2H6 and delta D-CH4 values is a good measure of the organic matter input of source rock. The value for marine sapropelic gas is normally < 12 and that for terrigenous humic gas > 10. The hydrogen isotopes of methane in Upper Palaeozoic natural gas was closely related to the vitrinite reflectance (% Ro) of the Upper Palaeozoic source rocks, displaying a short migration and accumulation feature. Sulige gas field is continually charged with coal-derived gas at different thermal maturation stages and the carbon and hydrogen isotopic reversals of alkanes result from the continuous charging and near-source migration and accumulation. (C) 2014 Elsevier Ltd.
Geochemical characteristics of the natural gas from Xujiahe Formation in the central Sichuan Basin, China
Geochemical characteristics of marine and terrestrial shale gas in China
DOI:10.1016/j.marpetgeo.2016.04.027 URL [Cited within: 3]
Origins of partially reversed alkane δ13C values for biogenic gases in China
DOI:10.1016/j.orggeochem.2004.01.006
URL
[Cited within: 1]

Abstract
With increasing molecular weight, δ13C values of hydrocarbon gases change in two different manners: the normal order would be δ13C1<δ13C2<δ13C3<δ13C4, whereas the reversed order would be δ13C1>δ13C2>δ13C3>δ13C4. Partially reversed order is common in gas samples from sedimentary basins in China, which can be attributed to one or several of the following four mixing processes: (a) mixing of biogenic and abiogenic gases; (b) mixing of sapropelic and humic sourced gases; (c) mixing of gases from the same types of source rocks with different maturity; and (d) mixing of gases from the same source rock interval of varying maturity.
Carbon isotope fractionation mechanism of alkane gases in Fischer- Tropsch synthesis experiments
Fische-|Tropsch synthesis experiments indicate that carbon isotopes of alkane gases reversed in these experiments on occasion. By analyzing the whole process and data of all researchers,the probable interpretations are deduced as fellows. As the products conversion heightening with the experiment time and temperature increasing,the control of alkane carbon isotope fractionation in the experiments was gradually shifted from kinetic mechanism to thermodynamic equilibrium. Series of carbon isotopes of alkane gases were changed from reversed to normally distribution just like alkanes in natural gas. The experiments were controlled by kinetic fractionation on the conditions of short time reaction (low conversion) or in an opened system (discharging following the reaction). And the electric spark synthesis experiment was more an ideal condition delegating the course of carbon isotope fractionation.
Carbon isotope fractionation in the Fischer-Tropsch synthesis and in meteorites
DOI:10.1126/science.170.3961.980
URL
PMID:17834614
[Cited within: 2]

Carbon dioxide and organic compounds made by a Fischer-Tropsch reaction at 400 degrees K show a kinetic isotope fractionation of 50 to 100 per mil, similar to that observed in carbonaceous chondrites. This result supports the view that organic compounds in meteorites were produced by catalytic reactions between carbon monoxide and hydrogen in the solar nebula.
The simulation experiment on gas-generating potential of over mature source rocks
As for the gasgenerating potential at the overmature stage, the authors used the RockEval rock pyrologger and TGMS hightemperature pyrogenating simulated experiment device to carry out a series of experiment and research. By using the coal and carbonaceous mudstone of Jurassic in Junggar Basin and outcrop coal sample of the Permian system and Carboniferous carbonaceous mudstone in Ordos Basin, we carried out the gasgenerating simulation experiment and the temperature is from 300℃ to 800℃. According to the result, all the rocks have generated a lot of gas when the temperature is lower than 600℃(that is Ro<2.0%~2.5%). When increasing the experiment temperature from 600℃ to 800℃, which means to raise Ro from 2.0%~2.5% to 5.0%, we found that both the coal and the carbonaceous mudstone in the two Basin can generate a great amount of natural gas between 600~800℃. The gas generated from the carboniferous carbonaceous mudstone accounts for 25.38%, 34.39%. The gas generated from the two type source rocks at the overmature stage accounts for 20%~35% of the total generated gas volume. In an attempt to verify the above phenomenon, we have cooperated with Taiyuan Institute of Coal Chemistry Chinese Academy of Sciences to carry out the source rock thermogravimetricmassspectrometric thermal simulated experiment and have got very similar result.According to the result of thermogravimetricmassspectrometric experiment on the shale of sea facies and the mud stone of lake facies, the two samples of Songliao Basin, the organic matter of Ⅱ2 residue gasgenerating when the temperature is between 600~800℃ accounts for 7% of the total generated gas volume or so and the organic matters of Ⅱ1 organic matter accounts for 5%. However, the gasgenerating volume is much smaller than the coalrelated source rock. There is a tendency in which the type of organic matter is worsening and the residue gas volume is reducing at high temperature, which is similar to that experiment result on the abovementioned pyrogenating gas generation rate. When the temperature is between 800~1000℃, the gasgenerating potential disappears, which is consistent with the experiment result of coalrelated source rock. Therefore, a large amount of natural gas can be generated at the overmature stage(Ro≥2.5)for coal and carbonaceous mudstone, and the volume can account for 20%,or even more. The low limit of Ro extend to 5.0% . The generated gas volume stops increasing when the temperature is larger than 800℃, so the simulation temperature 800℃ will be enough for the gasgeneration reflection.
New insights into secondary gas generation from the thermal cracking of oil: Methylated monoaromatics. A kinetic approach using 1, 2, 4-trimethylbenzene. Part I: A mechanistic kinetic model
DOI:10.1016/j.orggeochem.2009.10.013
URL
[Cited within: 1]

Abstract
The scope of the present study was to characterize the pathways leading to CH4 generation during the thermal degradation of a model compound representative of methylated monoaromatic hydrocarbons present in many crude oils. 1,2,4-trimethylbenzene was selected and subjected to pyrolysis experiments from 395 – 450 °C (at 100 bar). The whole range of reactant conversions was studied. All pyrolysis fractions were recovered and quantified. All products that could be quantified individually were used to develop a mechanistic kinetic model of 122 reversible reactions involving 47 species up to C18. The model was validated on our experimental results for conversions below 70%. The model was then used to assess the relative contributions of specified CH4 generation pathways at high (425 °C) and low (200 °C) temperatures. For the present case study, it was demonstrated that the pathways accounting for CH4 generation were the same at both temperatures. At low conversions, CH4 was mainly generated by the ‘polyaromatic route’, i.e. the dimerization of monoaromatics (step 1) followed by the intramolecular ring closure of these dimers (step 2). The contribution of the ‘monoaromatic route’, i.e. the successive demethylation reactions of methylated monoaromatics, was lower but not negligible. At higher conversions the contribution of the ‘monoaromatic route’ increased, eventually accounting for around 50% of the overall yield of CH4 at 70% conversion. Within the ‘polyaromatic route’, step 1 and step 2 quickly exhibited similar contributions, eventually sharing the remaining 50% of the overall CH4 yield at 70% conversion.
New insights into secondary gas generation from the thermal cracking of oil: Methylated monoaromatics. A kinetic approach using 1, 2, 4-trimethylbenzene. Part III: An isotopic fractionation model
DOI:10.1016/j.orggeochem.2010.02.008 URL [Cited within: 1]
Carbon isotope fractionation during shale gas transport: mechanism, characterization and significance
DOI:10.1007/s11430-019-9553-5 URL [Cited within: 2]