Introduction
Shales, sedimentary rocks constituted mainly by clays, it represents one of the greatest technical challenges in drilling operations when water-based drilling fluids (WBDF) are employed due to its hydration and swelling. Shales with high contents of hydratable clays (smectites), when in contact with the drilling fluid, can swell and disintegrate, weakening the well walls, which in extreme cases can lead to the collapse of the well and, therefore, to the loss of the drilling assembly, well side-tracks or definitive well abandonment[1]. In addition, the incorporation of fine solids from these shales negatively impacts the WBDF flow properties, increasing the costs associated with their reconditioning[2]. In order to minimize these problems, water-soluble inhibitors are frequently used. They interact with the shale formations and the cuttings generated by the bit, keeping them intact and compact.
Polyols represent the only class of nonionic shale hydration inhibitors that have been extensively used in WBDF. The generic name of polyols is given to a broad class of chemicals that include polyglycerols and polyalkylene glycols (PAGs) such as poly (ethylene glycol) (PEG), poly (propylene glycol) (PPG), among others, and copolymers of these (ex: PEG-PPG). PAGs were proposed as lubricants and shale stabilizers in the 1980s[3], and several WBDF formulations containing PAGs have been patented[4⇓-6]. The PAGs may play multiple roles such as drill lubrication, bit balling prevention, loss circulation control and shale hydration inhibition when be used as additives in WBDF due to their low cost, chemical and thermal stability, as well as their biodegradable, non-toxic and non-corrosive characteristics[7-8]. As shale stabilizers, they have shown a better performance in the presence of KCl[9-10]. Furthermore, it is desirable to have their molar mass lower than 10 kg/mol[11], since PAGs with higher masses are screened out on the shale surfaces, instead of getting into the system of pores that would give access to the clay fabric.
Generally, the two kinds of mechanism of action of PAGs as shale hydration inhibitors can be described as: i) Mechanism through TAME (thermally activated mud emulsion). This proposal is based on the property that PAGs have in aqueous solution to become cloudy, forming micro-droplets when reaching a critical temperature called CPT (cloud point temperature). The emulsions once formed would block the passing of the aqueous filtrate into the formation[11]. ii) Mechanism by adsorption of the inhibitor agent in the interlamellar region of expandable clays. The mechanism proposes that PAGs compete with water to adsorb on the active sites of the clay surface and interact with hydratable interlamellar cations. According to this hypothesis, PAGs would displace the water, inhibiting the hydration and swelling of clays[1]. Due to the fact that PAGs can stabilize shales even below the CPT, it suggests that the adsorption-based inhibition mechanism plays a determining role in the inhibition process.
Some researchers tried to establish relationships between structural characteristics of the molecular systems studied and the hydration inhibitory effect, to improve the performance of additive families by alternately combining hydrophilic and hydrophobic segments made up of monomeric or oligomeric segments to improve the inhibitory performance of PAGs[12⇓-14]. Also by the introduction of hydroxyl groups between hydrophobic segments[13] or by functionalization with intermediate[15-16] and terminal amine groups[17⇓-19]. A recent strategy, presented as environment friendly, proposes the use of ionic liquids based on PEG, which are adsorbed in the interlaminar region and on the external surface of the clays, efficiently blocking the inhibition of shale hydration[20].
Using another approach, previous studies carried out by our group have shown that the inhibitory performance of poly (ethylene glycol) oligomers is improved by the insertion of terminal hydrocarbon segments and by the presence of K+ cations[10,21]. However, the aspects involved in the clay-K+-hydrophobized PEG interaction are still neither fully understood nor how this interaction results in the inhibition of the shales hydration. The present work experimentally studied the mechanism of clay-K+-hydrophobized PEG interaction and how this interaction results in the inhibition of the shales hydration. As the standards from reference[21] three molecular systems based on PEG2000 were chosen whose structure was systematically modified to observe the effect due to hydrophobization. The criteria used for the choice of these systems are given in our previous work[21]. The study was conducted through performance tests for inhibitor, and the modification of cuttings hot-rolling dispersion test was designed to evaluate the effect of CPT on the cutting's recovery. To the best of our knowledge, this is the first time that the test is adapted to show a possible inhibitory effect through TAME. In order to correlate structural aspects of the hydrophobized PEG/K+ with the performance results, clay-K+-PEG interaction studies were performed through solid-liquid adsorption and contact angle measurements, combined with X-ray diffraction, Fourier transform infrared spectroscopy and thermogravimetric analysis. Key observations and conclusions regarding the role of hydrophobic tails and K+ were obtained by comparing them with the results of the previous complementary study conducted in the absence of K+[21].
1. Experiment
1.1. Materials
Two types of clay materials (Calumbi shale and bentonite) were used in this study. Samples of Shale were collected from a natural outcrop of Calumbi shale, located in the Sergipe State, Brazilian. Bentonite, PEG2000 and poly (ethylene glycol) methyl ether 2000 (mPEG2000) were provided by Sigma-Aldrich, Brazil. Oleic and p-toluenesulfonic acids, toluene, potassium chloride (KCl) and sodium hydroxide (NaOH) were purchased from Vetec Química, Brazil. NaOH was used in the experiments to adjust the pH to 10, in order to better represent the characteristics of the WBDF environment. mPEG2000 monooleate (mPEG2000-OA) and PEG2000 dioleate (OA-PEG2000-OA) were synthesized according to the procedure from reference [21]. Table 1 shows the structure and molecular weights of the inhibitors. The PEG presented in following of the paper is a general term for PEG2000 and two kinds of hydrophobic products.
Table 1. The structure and molecular weights of the shale inhibitors.
| Inhibitors | Structure | Molecular weights/(g•mol-1) |
|---|---|---|
| PEG2000 | 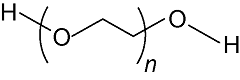 | 2 000 |
| mPEG2000-OA | 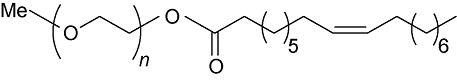 | 2 264 |
| OA-PEG2000-OA | 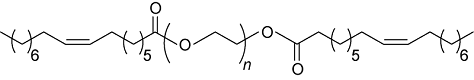 | 2 528 |
1.2. Methods
1.2.1. Determination of cloud point temperature (CPT)
100 mL of aqueous solutions of mPEG2000-OA/KCl and OA-PEG2000-OA/KCl were prepared respectively, with each solute at 30 g/L. With an adapted thermometer in the liquid, the solutions were slowly heated under magnetic stirring. The temperature at which the solution began to turn cloudy was recorded. The procedure was repeated three times and the average of the measurements was considered.
1.2.2. Cuttings hot-rolling dispersion test
Two different sets of tests were performed: a conventional test that was conducted following the general procedure described by reference [22], and a modified version of the test in which the temperature was varied.
Conventional test: was conducted taking 50 g of Calumbi shale, previously granulated and selected between 2.36-4.75 mm (4-8 mesh) sieves. The cuttings were added to stainless steel aging cells containing 350 mL of inhibitor system (aqueous solutions containing PEG at 0, 5, 10, 20, 30 40 or 50 g/L, and KCl at 30 g/L). The pH was adjusted to 10 and then, the samples were heated in a roller oven at 66 °C for 16 h.
Modified test: 50 g of shale cuttings, previously selected with 2.36-4.75 mm (4-8 mesh) sieves, were hot-rolled, with different temperature programs (28, 66, 80 and 90 °C), in 350 mL of inhibitor system (aqueous solutions containing PEG and KCl, each at 30 g/L). The experiments at 28 and 66 °C were carried out in the same way as in the conventional test. On the other hand, in the experiments at 80 and 90 °C, the solution was heated and just 1 °C before the oven temperature reached the CPT, the 50 g cuttings were added and the heating was kept for 16 h. To ensure accurate temperature measurements the digital roller oven thermometer was calibrated with the thermometer used to measure the CPT of the inhibitor solutions.
The material resulting from all the tests was dried at 80 °C for 16 h and then classified and separated using 0.60 mm (30 mesh) and 2.36 mm (8 mesh) sieves to determine the mass of total and intact cuttings recovered, respectively. The mass fraction of recovered cuttings was determined as described in the Eq. (1), where w0 and w represent, respectively, the initial and final cutting masses recovered.
1.2.3. Bentonite inhibition test
Following the study procedure described by Patel et al.[23], while shearing, 2.5 g of bentonite was added to 350 mL of inhibitor system (aqueous solutions containing PEG at 0, 5, 10, 20, 30, 40 or 50 g/L, and KCl at 30 g/L). Having adjusted the pH to 10, the mixture was stirred for 30 min and transferred to a roller oven in stainless steel aging cells. After heating at 66 °C for 16 hours, the suspensions were cooled to room temperature and the yield point (YP) was determined with a Fann 35A viscometer. A new amount of bentonite was added, and the previous procedure repeated for several times until the upper reading limit of the viscometer was reached. The YP was calculated from the readings at 600 ( ϕ600) and 300 (ϕ300) r/min using the following approximate formula:
1.2.4. Adsorption measurements
Aqueous solutions containing both PEG (0.005-4.500 g/L) and KCl (30 g/L) were prepared with ultrapure water, obtained from a MilliQ filtration system. Next, 70 mL of solution were added to Erlenmeyer flasks containing 0.5 g of bentonite. A control sample containing just water and clay was included. Having adjusted the pH to 10, the suspensions were equilibrated in a reciprocating shaker at 180 cycles/min for 24 h, in a thermostatic bath at 25 °C. The samples were centrifuged at 4000 r/min for 40 min. An aliquot of each original solution and each corresponding supernatant was collected and diluted 10 times to determine the initial ( Ci) and the equilibrium (Ceq) PEG concentration, respectively, with the aid of a total organic carbon analyzer TOC-LCPN(Shimadzu, Japan). The difference between Ci and Ceq allowed the determination of the PEG amount adsorbed (Presented by the ratio of PEG amount adsorbed to bentonite consumption) for each equilibrium point, as described in Eq. (3). The remaining solid after centrifugation was washed twice, dried at 90 °C for 16 h, and then pulverized. The obtained samples were stored in a desiccator and used to conduct further analysis.
1.2.5. Contact angle measurements
PEG was adsorbed on bentonite at surface saturation for 24 h. The dispersions were used to coat the glass slides following the procedures described by Wu[24]. The glass slides were allowed to air dry. Contact angles were determined with the sessile drop method using a contact angle goniometer.
1.2.6. Fourier transform infrared spectroscopy (FT-IR) analyses
FT-IR measurements were performed in a Magna-IR 740 spectrometer (Nicolet, USA). The spectra were collected in the range of 400-4000 cm-1 with a resolution of 4 cm-1 and a total of 16 scans. After correcting the baseline, the spectra were normalized for the most intense band located around 1045 cm-1, representing the stretching of the Si-O bond.
1.2.7. X-ray diffraction (XRD) analyses
XRD analyses were performed using a Siemens D5005 diffractometer (Bruker-AXS, Germany), with incident ray wavelength λ= 1.54mm, operating at 40 kV and 20 mA. The data were collected over a 2θ range of 2° to 30°, with a step size of 0.02° 2θ counted for 10 s. The bentonite interlamellar spacingd001-value was calculated according to Bragg's Law:
1.2.8. Thermogravimetric analyses (TGA)
TGA were performed in a SDT Q600 V20.9 Build 20 instrument (TA Instruments, USA), with a heating ramp of 10 °C/min from room temperature to 900 °C, in a flowing dry nitrogen atmosphere (20 cm3/min).
1.2.9. Water-based drilling fluids (WBDF) formulation and properties determination
Based on a typical formulation, three WBDF (F1, F2 and F3) and a blank fluid (BF) were prepared (Table 2). The fluids were prepared making use of a Hamilton Beach three speed mixer under stirring speed of 13 000 r/min. Initially, 250 mL of water was mixed with xanthan gum (XG) for 10 min. Thereafter, and under continued stirring, the other additives were introduced at intervals of 5 min in the following order: hydroxypropyl starch (HS), KCl, NaOH, BaSO4, CaCO3 and, at last, PEG previously dissolved in 100 mL of water. The fluids were aged in Baroid cells at 66 °C for 16 h. In the aging process, 50 g of cuttings were added to each fluid in order to determine the ability to inhibit hydration and cuttings disintegration. The pH and density, as well as the viscosity, the filtration behavior and lubricity were measured. The properties determinations were performed according to the recommended standard procedures from the American Petroleum Institute API[25]. The viscous flow parameters were determined in a Fann 35A viscometer; the filtration test was conducted in a HTHP Filter Press Series Fann 387, at 25 °C, 0.69 Mpa (100 psi) for 30 min; and lubricity test was performed in a Lubricity Tester Fann 21200.
Table 2. Composition of WBDF and blank control solution.
| Experimental fluid | Additive mass/g | ||||||
|---|---|---|---|---|---|---|---|
| Xanthan gum | Hydroxypropyl starch | PEG | KCl | NaOH | BaSO4 | CaCO3 | |
| BF | 1.2 | 8 | 0 | 0 | 0.5 | 30 | 9 |
| F1 | 2.0 | 8 | 10.5 (PEG2000) | 10.5 | 0.5 | 30 | 9 |
| F2 | 1.2 | 8 | 10.5 (mPEG2000-OA) | 10.5 | 0.5 | 30 | 9 |
| F3 | 2.0 | 8 | 10.5 (OA-PEG2000-OA) | 10.5 | 0.5 | 30 | 9 |
Note: The volume of water solution is 350 ml.
2. Results and discussion
2.1. Inhibitory performance test
2.1.1. Conventional cuttings hot-rolling dispersion test
This test determines directly the efficiency of inhibitors in maintaining the integrity of the cuttings released from the drilling action, and the results are also often used as an indication of the inhibitive capacity to minimize interaction of the aqueous filtrate with the shale formation. The greater the efficiency of the inhibitor, the greater the quantity and the better is the size preservation of the cuttings recovered in the test.
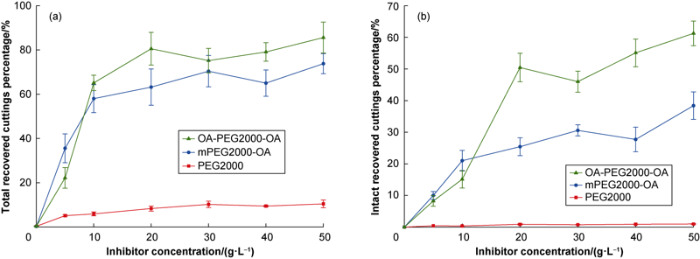
This test evaluates the efficiency of the PEG/K+ systems in maintaining the integrity of the shale cuttings in aqueous solution. To make WBDF get high inhibitor capacity, the PEG concentration was varied between 0 and 50 g/L while the concentration of KCl was set at 30 g/L according to previous researches[9-10,26 -27]. The calculation results of mass percentage of the intact and the total shale cuttings recovery (Fig. 1), showed that all PEG had the ability to inhibit the dispersion of shales, exhibiting different degrees of inhibition ability, OA-PEG2000-OA had the best inhibition ability, mPEG2000-OA was the second, and PEG2000 was the last. These results contrast with those of the previous work carried out in the absence of KCl where none of the products showed inhibitory capacity[21], all PEG had the ability to inhibit the dispersion of shales, showing different degrees of inhibition ability, OA-PEG2000-OA had the best inhibition ability, mPEG2000-OA was the second, and PEG2000 was the last. These observations allowed to infer that the inhibition of hydration and disaggregation of shales occur as a consequence of a synergic action between PEG and K+, the hydrophobic segments (the hydrocarbon chain of oleic acid linked to the PEG backbone) being decisive to enhance the performance. In all cases, the inhibitory capacity increased with the concentration of PEG. The recovery percentages tended to stabilize at inhibitor concentrations above 10 g/L, when the molecular systems would be reaching their maximum adsorption on shale cuttings matrix.
Fig. 1.
Fig. 1.
The cuttings recovery percentages of 3 inhibitors obtained from conventional hot-rolling dispersion test (the line segment in the curve represents the standard deviation).
The above findings were ratified with contact angle measurements performed through the sessile drop method on films prepared with bent/K+/PEG dispersions at surface saturation. The contact angle of the untreated bentonite was 24.7°, very close to the prevenient experimental results (contact angle was 23.8°)[28]. The contact angles measured on the bent/K+/PEG films increased with the hydrophobicity of the PEG. The contact angles of PEG2000, mPEG2000-OA and OA-PEG2000-OA were 31.9°, 43.2° and 57.6°, respectively.
The performance of two recognized cationic inhibitors, Jeffamine D-230 and poly (diallyldimethylammonium chloride) (PDADMAC) was also evaluated. The results showed that the percentage of total and intact recovered cuttings with mPEG2000-OA/K+ and OA-PEG2000- OA/K+ (Total recovered cuttings percentages were 70.4%, 75.2%; intact recovered cuttings percentages were 30.6%, 46.0%) were comparable to that of Jeffamine D-230 and PDADMAC (Total recovered cuttings percentages were 65.2%, 80.3%; intact recovered cuttings percentages were 30.0%, 54.7%), respectively. It showed that the inhibition ability of this system is good.
2.1.2. Modified cuttings hot-rolling dispersion test
The modified cuttings hot-rolling dispersion test was designed to evaluate the effect of CPT on the performance of hydrophobized PEG, considering that PEG could perform through TAME mechanism. The PEG2000 hydrophilic system was not evaluated for reasons of experimental limitations since its high CPT (higher than 100 °C) would make it difficult to heat. The CPT of the evaluated ternary mixtures (water/PEG/KCl) was determined through visual inspection, which has demonstrated high precision when compared with the diffractometric method[29]. The CPT values found were 77 and 71 °C for the aqueous systems mPEG2000-OA/KCl and OA-PEG2000-OA/KCl, respectively, each solute at 30 g/L. The purpose of the modified hot rolling dispersion test was to evaluate the recovery of cuttings in a set of temperatures that will cover points below and above the CPT. Thus, four temperatures were arbitrarily selected, 28 °C, 66 °C, 80 °C and 90 °C.
It seems reasonable to think that if the clouding of inhibitors has an inhibitory effect, it would result in an increase of cuttings recovery in those experiments performed at a temperature higher that the CPT. However, no increase in the recovery percentages was observed. On the contrary, inhibitory performance tended to decrease with increasing temperature (Fig. 2). Thus, inhibition through TAME was not found to occur under the experimental conditions of cuttings hot-rolling dispersion test.
Fig. 2.
Fig. 2.
The cuttings recovery percentages of 2 inhibitors at different temperature in Modified cuttings hot-rolling dispersion test.
2.1.3. Bentonite inhibition test
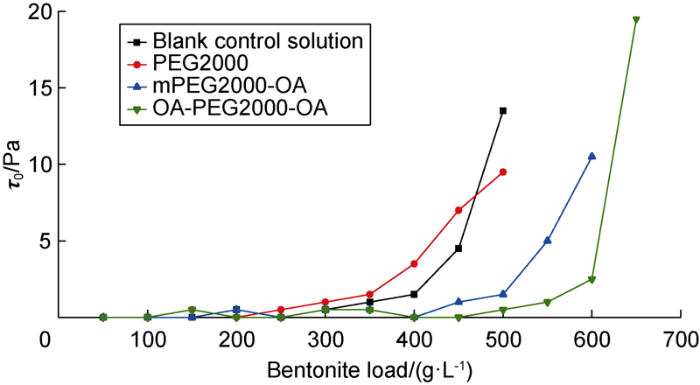
The continuous incorporation of fine material during the drilling of shale sections can negatively impact the flow properties of a WBDF. Clay fines, when in contact with water, swell and disperse forming a three-dimensional network that increases the viscosity of the fluid as a function of the number of particles and the strength of the interactions. Thus, the suppression of the viscosimetric parameters (yield point (YP)), reflects the ability of the tested product to prevent hydration and dispersion of clay particles. The inhibition effect was proved to be good even with a large bentonite load under low YP. (Bentonite load indicates the addition amount of bentonite exceed the initial amount of 2.5 g). It can be seen from Fig. 3 that the inhibition performance of PEG2000/KCl aqueous solution is not improved compared with the blank control solution containing KCl only, but both hydrophobic products show better inhibition performance.
Fig. 3.
Fig. 3.
Yield point of bentonite/PEG/KCl suspensions as a function of bentonite loading. In PEG / KCl system, the mass concentration of PEG and KCl was 30 g/L;The KCl concentration of blank control solution was 30 g/L.
The incorporation of hydrophobic chains of oleic acid to PEG2000 backbone suppressed the YP of bentonite suspensions, and improved inhibition ability of molecular system. The inhibitory contribution made by the K+, due to its binding capacity of the clay particles, explains the higher bentonite loads supported by these systems evaluated in the KCl presence, compared with the studies results conducted in the absence of this salt[21] (Table 3).
Table 3. Bentonite loading of water dispersions containing inhibitors under different conditions.
| Water dispersion system | Bentonite load /(g•L-1) | |||||
|---|---|---|---|---|---|---|
| Control group | PEG2000 | mPEG2000- OA | OA- PEG2000-OA | Jeffamine D-230 | PDADMAC | |
| No KCl | 40* | 60* | 100* | 200* | 120 | 650 |
| KCl | 400 | 350 | 500 | 600 | / | / |
Note: The bentonite load in the table refers to the corresponding bentonite load before the sudden increase of YP; * representative data are from reference [
mPEG2000-OA/K+ and OA-PEG2000-OA/K+ systems showed performances close to those of reference inhibitor PDADMAC; mPEG2000-OA and OA-PEG2000-OA systems showed performances close to those of reference inhibitor Jeffmine D-230 (Table 3)
2.2. Adsorption isotherms
The adsorption behavior of the different molecular systems is shown by the adsorption isotherms. Langmuir model was used to fit experimental data by Eq. (5). The higher the value of KL, the higher the affinity of the inhibitor to clay surface. Values of the Langmuir parameters obtained from the fittings are presented (Table 4).
Table 4. Langmuir simulation parameters and correlation coefficients of adsorption experiment with or without KCl.
| PEG | K+ | No K+ | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Qmax | KL | R2 | Qmax | KL | R2 | |||||
| mg/g | μmol/g | L/mg | L/μmol | mg/g | μmol/g | L/mg | L/μmol | |||
| PEG2000 | 210.8 | 105.4 | 2.963×10-1 | 5.924×10-1 | 0.854 2 | 246.5 | 123.2 | 6.27×10-3 | 12.54×10-3 | 0.924 8 |
| mPEG2000-OA | 262.9 | 116.1 | 2.591×10-2 | 5.867×10-2 | 0.972 1 | 282.2 | 124.6 | 8.59×10-3 | 19.45×10-3 | 0.915 1 |
| OA-PEG2000-OA | 292.1 | 115.5 | 4.782×10-1 | 1.209×10-1 | 0.994 9 | 309.6 | 122.4 | 5.98×10-3 | 15.13×10-3 | 0.957 4 |
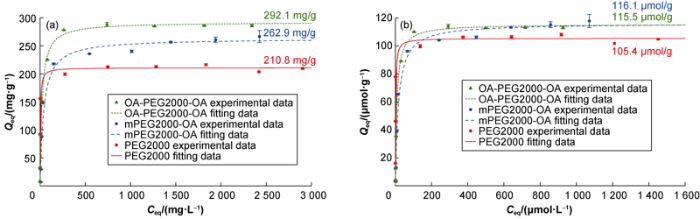
The Qmax value, in terms of mg/g, increased with the molar mass of the adsorbed PEG (Fig. 4a); However, in terms of mol/g, there were just a slight superiority for the hydrophobic products (Fig. 4b), This indicates that the number of molecules adsorbed at surface saturation is almost the same in all cases, which suggests that the PEG2000 backbone, molecular segment that remains invariable in the three systems studied, is the one responsible for the adsorption process on the bentonite surface. The hydrophobic tails would be intervening little or nothing in this process. Similar results were found in the absence of KCl[21].
Fig. 4.
Fig. 4.
Adsorption isotherms of PEG on bentonite, in the KCl presence.
On the other hand, the K+ presence increases the affinity of the molecular systems for the surface of the bentonite. The KL values are higher, by one or two orders of magnitude, for the systems obtained here, compared to those found in the absence of K+ (Table 4). The adsorption curves showed that the curves sharpest rise, before reaching the plateau. Previous studies have shown that K+ has great affinity both for the clay surface and for the PEG backbone oxygen atoms[30⇓⇓-33]. The joint interpretation of these results with the adsorption ones obtained here, suggests that the formation of clay/K+/PEG complexes increase PEG2000 backbone affinity for the ben-tonite, and efficiently reduce the clays hydration and consequent swelling.
2.3. Fourier transform infrared spectroscopy (FT-IR) analyses
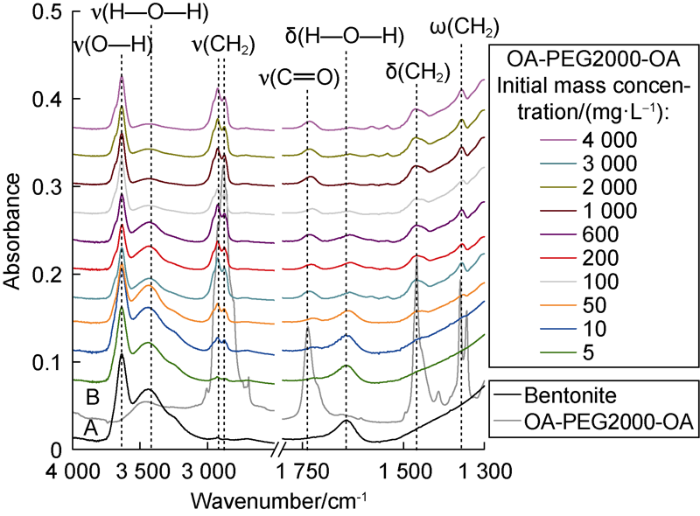
To understand the effect of the clay/K+/PEG complexes on the clay hydration inhibition, the solids obtained from the adsorption studies were studied by FT-IR. As an example, a comparison of the spectra of bentonite, OA-PEG2000-OA and bent/K+/OA-PEG2000-OA complexes, are presented (Fig. 5). The spectra A and B correspond respectively to bentonite and OA-PEG2000-OA. The remaining spectra correspond to the bent/K+/OA- PEG2000-OA complexes obtained in the adsorption experiments. The intensity of the bands related to stretching, bending, wagging vibrations of CH2 groups and stretching vibrations of the C=O group in the PEG increased as the concentration of this increased in the adsorption test. In addition, the decrease in band intensity of H—O—H stretching and bending vibration showed that the adsorption of polymer cut down the water content in clay and promoted the water displacement. To surface saturation (top spectra in Fig. 5), the formation of bent/K+/PEG complexes displaced, almost completely, the water of the bentonite interlamellar region. Similar trends were found in the comparison made for the bent/K+/PEG2000 and bent/K+/mPEG2000-OA complexes. A detailed description of the FT-IR bands associated with the PEGs studied and bentonite was given by reference [21].
Fig. 5.
Fig. 5.
FT-IR spectra.
ν—Stretching vibration; δ—Bending vibration; ω—Wagging vibration
The comparison of the bands associated with the hydration water in bentonite and complexes at surface saturation reveal the capacity of each inhibitor system to reduce the bentonite hydration (Fig. 6). The A and B series spectra correspond respectively to bentonite and bent/PEG saturated complexes, and the C series spectra correspond to bent/K+/PEG saturated complexes. The numbers associated with each series represent the type of PEG in the complex: (1) PEG2000, (2) mPEG2000-OA and (3) OA-PEG2000-OA. Within each series of spectra (B or C), the water content tended to decrease with the increase in the hydrophobicity of the PEG. For complexes formed by the same PEG type (ex: B1 and C1), the complexes with K+ (C series) exhibited considerably lower intensity bands. Therefore, the formation of bent/K+/PEG complexes was more efficient in reducing the hydration of the clay than the formation of bent/PEG complexes. These observations are consistent with the test results of bentonite inhibition.
Fig. 6.
Fig. 6.
Region of the FT-IR spectra for: (a) stretching and (b) bending vibration area.
Compared with untreated bentonite (A series), the shifting of the vibration modes ν (HOH) and δ (HOH), towards lower and higher frequencies, respectively in Bent/PEG complexes (Fig. 6, B series), were attributed to a reduction in the polarization of the O-H bond and the increase of the hydrogen bond network. Water molecules solvate the interlamellar cations (Na+, Ca2+), and interact with the oxygen atoms of the intercalated PEG molecules[21]. In this scenario, no direct association between the cations and the PEG chain would be occurring. In the presence of K+, changes in the shifting patterns, suggesting a different PEG-cation interaction mode than the "water bridges" described[21]. In the bent/K+/PEG complexes (C series), both vibration modes experienced discrete displacements towards lower frequencies. The observation is consistent with the direct coordination between K+ cations and oxygen atoms of the PEG chains, which leads led to a dramatic reduction in water content. The little water that remained could have been confined in small clusters associated with Na+ and Ca2+ cations that were not exchanged with K+. This new condition led to the energy of the vibration mode ν (HOH) being closer to that of the bentonite, and that the vibration energy associated with the mode δ (HOH) reaching a minimum value. This status responded to an extreme impoverishment of the hydrogen bond network.
In the region of the -OH groups of the siloxane lattice, the spectra of all the complexes exhibited a shoulder around 3680 cm-1, which was not detected in the unmodified bentonite (Fig. 6a). Therefore, this signal cannot be attributed to the structural Al-OH group of the bentonite, but to an interaction of it, on edge surfaces, with the molecular system adsorbed[34]. This observation was reported in previous studies and was attributed to interactions between the -OH groups and the PEG backbone oxygen atoms, via hydrogen bonding[21,35].
2.4. X-ray diffraction
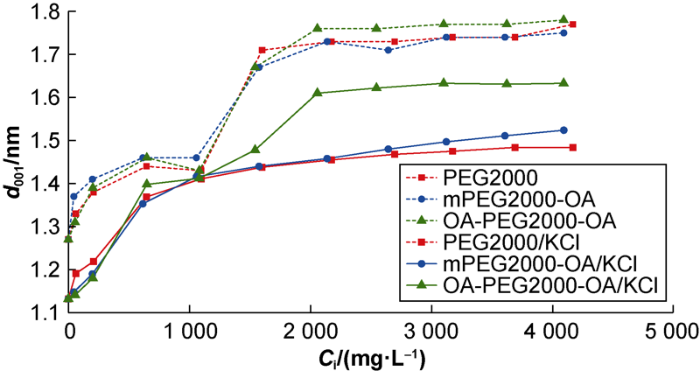
The bent/K+/PEG complexes were analyzed through XRD to determine the effect of the PEG/K+ inhibitor system on the d001-value of the bentonite interlamellar spacing. The XRD patterns obtained from the test show that: (1) the modified bentonite exchanged with K+ exhibited a diffraction peak at 2θ=7.54°, which corresponded tod001-value of 1.17 nm, showing that it was less expanded than the unmodified bentonite[21]. A monolayer of K+-hydrate is consistent with the d001-value found[36]; (2) The interlamellar spacing increased as a function of the concentration of PEG introduced in the adsorption test, indicating that the molecular systems were intercalated; (3) A reflection of greater intensity and smaller full width at half-height is evidenced, at the same PEG concentration, in the range of 2θ=6.20-6.26°, corresponding tod001-value between 1.41 and 1.42 nm. These d001-values are close to those found (1.44-1.46 nm) in the study performed in the absence of K+[21]. On the other hand, the XRD patterns differ in the PEG/K+ inhibitor system at surface saturation, the d001-value increase as a function of the PEG hydrophobicity, the d001-values of PEG2000/KCl, mPEG2000-OA/KCl and OA-PEG2000-OA/KCl inhibitor systems are 1.48 nm, 1.52 nm and 1.63 nm, respectively. To visualize the evolution of the interlamellar spacing, the d001-value was plotted as a function of the initial concentration of PEG used in the adsorption test (Fig. 7). The systems containing K+exhibited the lowest expansions at surface saturation.
Fig. 7.
Fig. 7.
Variation of d001-value in the complexes with initial concentration of PEG.
In the absence of K+, it was found that at a concentration of 1000 mg/L the PEG molecules were intercalated in the clay minerals interlamellar region forming monolayers (The curve presents a platform segment), while at surface saturation they formed bilayers (The curve presents the second platform segment) (Fig. 7)[21]. In the presence of K+, a partially similar behavior is observed. Considering that the interlamellar spacing of the K+-Bent in the dehydrated state is 1nm[37], the spacing of ~1.4 nm reached at 1000 mg/L, correspond to an increase in the interlayer distance of ~0.4 nm, that provides the appropriate dimension for a PEG monolayer arrangement[38]. However, contrary to what occurred in the absence of K+, the expansions achieved at surface saturation provided increases in the interlamellar distance of 0.48 nm (PEG2000 systems), 0.52 nm (mPEG2000-OA systems) and 0.63 nm (OA-PEG2000-OA systems), insufficient to allow for a PEG bilayer arrangement (interlayer distance of ~0.8 nm), and this means that the inhibition performance is the best at this time. These results could be due to the direct coordination of the PEG backbone with the K+ cations located near the surface, on opposite sides of the interlamellar region, precluding the bilayers arrangement; in a way that resembles the interaction model with polycationic molecular systems, which keep the sheets together and offers resistance to swelling[18]. The model explains the synergism observed in the inhibition of swelling and disaggregation of shales. In this process, the hydrophobic tails anchored to the PEG chain play a crucial role, enhancing the ability to inhibit hydration.
2.5. Thermogravimetric analysis (TGA)
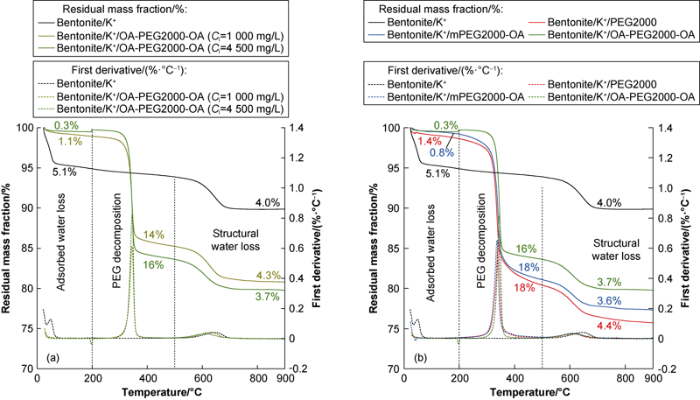
Thermogravimetric (TG) and the differential thermogravimetric (DTG) curves obtained for the different initial concentration bent/K+/OA-PEG2000-OA complexes and bent/K+/PEG saturated complexes from experimental results are presented (Fig. 8). All the complexes exhibit three stages of mass loss, which were attributed to the loss of adsorbed water (22-200 °C), PEG decomposition (200-500 °C) and structural water loss (500-900 °C).
Fig. 8.
Fig. 8.
TG and DTG profiles for different initial concentrations: (a) bentonite/K+/OA-PEG2000-OA complexes, (b) bentonite/K+/PEG saturated complexes (Ci=4500 mg/L).
The water content (5.1%) found in bentonite decreased for the complexes with the increase of the Ciaccording to TG curves (Fig. 8a). The inverse relationship between the water content and the mass loss associated with the PEG degradation showed that the water present in the bentonite is displaced as PEG molecules are adsorbed. Concerning the saturation of surface, the efficiency in the displacement of water has positive correlation with the hydrophobicity of the PEG (Fig. 8b), that is, with the improvement of inhibition performance, the water content of clay decreases. The water content of clay, clay/K+/ PEG2000, clay/K+/mPEG2000-OA and clay/K+/OA- PEG2000-OA decreases successively, which are 5.1%, 1.4%, 0.8% and 0.3%, respectively. These observations are consistent with the results of the contact angle measurements and FT-IR study.
3. WBDF properties determinations
In order to verify the inhibitory effect of the PEG/K+ system used in drilling operations in the field, the cuttings hot-rolling dispersion test was carried out using formulations of WBDF. Besides, related properties considered to guarantee the optimal general performance of WBDF.
Shale swelling involves the diffusion of fluid into the rock so that discrepant viscosities could lead to misleading assessments. In order to obtain viscosimetric parameters as close as possible, the dosage of xanthan gum was not the same in different drilling fluid (Table 2). As shown (Table 5), the WBDF systems showed the same inhibitory tendencies previously found, but with some enhancement, reaching cuttings recovery up to 99% partly. This was expected since effects such as the increase of the viscosity and reduction of water activity, due to the presence of other additives, maximizes the effect of the shale's stability[11]. It was also observed that the fluids adding inhibitors were able to efficiently maintain the flow properties when compared with the blank fluid. The other evaluated properties also showed to be within the acceptable ranges, indicating that WBDF containing hydrophobized PEG/KCl as inhibitor system can be formulated with the adequate properties that guarantee their good overall performance.
Table 5. Values of the properties evaluated in WBDF before and after aging.
| Water-base drilling fluids | Status | Performance | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total recovered cuttings percentage/% | Intact recovered cuttings percentage/% | Apparent viscosity/ (mPa•s) | Plastic viscosity/ (mPa•s) | Yield point/Pa | Initial gel strength/Pa | Final gel strength/Pa | Density/ (g•cm-3) | pH | API fluid loss/mL | Friction factor | ||
| BF | Before aging | 34.5 | 20 | 14.5 | 10 | 11.5 | 1.07 | 10.5 | 3.0 | 0.32 | ||
| F1 | 29.0 | 14 | 15.0 | 6.0 | 8.0 | 1.05 | 10.4 | 2.1 | 0.19 | |||
| F2 | 38.0 | 18 | 20.0 | 7.5 | 8.5 | 1.09 | 10.2 | 2.0 | 0.18 | |||
| F3 | 32.0 | 16 | 16.0 | 6.0 | 7.5 | 1.04 | 10.1 | 2.1 | 0.20 | |||
| BF | After aging | 4.9 | 0 | 54.5 | 26 | 28.5 | 11.0 | 13.0 | 1.10 | 10.5 | 2.4 | 0.34 |
| F1 | 80.2 | 71.5 | 35.0 | 17 | 18.0 | 6.5 | 8.0 | 1.10 | 10.2 | 2.0 | 0.19 | |
| F2 | 91.3 | 84.1 | 45.0 | 22 | 23.0 | 7.5 | 9.0 | 1.13 | 10.0 | 1.8 | 0.19 | |
| F3 | 99.2 | 89.9 | 36.0 | 18 | 18.0 | 6.5 | 7.5 | 1.08 | 10.0 | 1.9 | 0.20 | |
4. Conclusions
The mechanism of PEG/K+ system inhibits the hydration and disaggregation of shales are, (1) the presence of K+ conditions makes the intercalation of PEG to just one layer, (2) The hydrophobization effect of the mineral surface owing to adding PEG/K+ evidenced by the increase in the contact angle and the lower water content in the clay complexes, which were the key factors that keep the shale integrity.
An additional fact that the higher affinity of the polymer for the clay surface conferred by the presence of K+. This suggests that the intercalated PEG/K+ system performs as a pseudopolycationic molecular system. The cationic character would be given by the K+ ions that directly coordinate with the oxygen atoms of the etheric PEG chain. The region free of the K+ ions would in turn interact strongly with the negatively charged sites, in the ditrigonal cavities, on the opposite sides of the interlamellar surface precluding the PEG bilayers arrangement. This mode of action would result in a "zipper" effect, which would keep the sheets together, inhibiting the hydration and swelling of the shales when in contact with the aqueous medium.
The authors gratefully acknowledge to ANP (Brazilian Petroleum National Agency) and COLFUTURO (Foundation for the future of Colombia) for the financial support.
Nomenclature
Ceq—PEG concentration under condition of equilibrium, mg/L or μmol/g;
Ci—PEG concentration under initial conditions, mg/L;
d001—interlamellar spacing of Bentonite, nm;
KL—langmuir equilibrium constant, L/mg or L/μmol;
m—clay mass, g;
n—number of ethylene oxide units;
Qeq—adsorption quantity of PEG under condition of equilibrium, mg/g or μmol/g;
Qmax—the maximum Adsorption quantity of PEG when the surface is saturated, mg/g or μmol/g;
R—related coefficient;
w—recovered cuttings quantity, g;
wt—mass fraction of recovered cuttings, %;
w0—initial cuttings mass, g;
V—volume of solution, L;
τ0—yield point, Pa;
2θ—diffraction angle, °;
λ—wavelength, nm;
ϕ300—viscometer reading at 300 R/min, Pa;
ϕ600—viscometer reading at 600 R/min, Pa.
Acknowledgments
The authors gratefully acknowledge to ANP (Brazilian Petroleum National Agency) and COLFUTURO (Foun-dation for the future of Colombia) for the financial support.
Reference
Clay swelling: A challenge in the oilfield
DOI:10.1016/j.earscirev.2009.11.003 URL [Cited within: 2]
Designing and managing drilling fluid
Mechanism of shale inhibition by polyols in water-based drilling fluids
Hydrophobically modified poly(ethylene glycol) as reactive clays inhibitor additive in water-based drilling fluids
DOI:10.1002/app.v117:2 URL [Cited within: 3]
On the physical and chemical stability of shales
DOI:10.1016/S0920-4105(03)00034-2 URL [Cited within: 3]
Mechanisms of shale inhibition by polyglycol water-based muds and the development of improved additives through combined use of experimental and molecular modeling techniques
Molecular modeling of the mechanism of action of organic clay-swelling inhibitors
DOI:10.1080/08927020108023012 URL [Cited within: 2]
Swelling inhibition by polyglycols in montmorillonite dispersions
DOI:10.1081/DIS-120027669 URL [Cited within: 1]
Shale hydration inhibition characteristics and mechanism of a new amine-based additive in water-based drilling fluids
DOI:10.1016/j.petlm.2017.05.003 URL [Cited within: 1]
Evaluation of clay hydration and swelling inhibition using quaternary ammonium dicationic surfactant with phenyl linker
DOI:10.3390/molecules25184333 URL [Cited within: 1]
Rule based design of clay-swelling inhibitors
Structure-property relationship of polyetheramines as clay-swelling inhibitors in water-based drilling fluids
DOI:10.1002/app.38784 URL [Cited within: 1]
Biodegradable polyethylene glycol-based ionic liquids for effective inhibition of shale hydration
DOI:10.1039/C5RA02236C URL [Cited within: 1]
Understanding the clay-PEG (and hydrophobic derivatives) interactions and their effect on clay hydration and dispersion: A comparative study
DOI:10.1016/j.clay.2017.03.021 URL [Cited within: 16]
The development of an inhibitory cationic drilling fluid for slim-hole coring applications
Baseline studies of the clay minerals society source clays: Colloid and surface phenomena
DOI:10.1346/CCMN URL [Cited within: 1]
Standard procedure for field testing water-based drilling fluids: API RP 13B-1
Use of KCl-polymer clouding out polyol drilling fluid in combating high pressure in deep exploratory wells of Assam field: A case study
New multifunctional polymeric additives for water-based muds
Contact angles of aluminosilicate clays as affected by relative humidity and exchangeable cations
DOI:10.1016/j.colsurfa.2009.10.013 URL [Cited within: 1]
Cloud-point measurements for (water+poly(ethylene glycol)+salt) ternary mixtures by refractometry method
DOI:10.1021/je060061y URL [Cited within: 1]
Monte Carlo molecular modeling studies of hydrated Li-, Na-, K-smectites: Understanding the role of potassium as a clay swelling inhibitor
DOI:10.1021/ja00155a025 URL [Cited within: 1]
Conductometric study of the interaction of 1:1 electrolytes with nonionic surfactants having short polyoxyethylated chains: Methanolic solutions of H- and F-alkylated surfactants and oil/water microemulsions
DOI:10.1016/0021-9797(87)90102-0 URL [Cited within: 1]
Poly(oxyethylene)-cation interactions in aqueous solution: A molecular dynamics study
DOI:10.1016/S1089-3156(99)00015-X URL [Cited within: 1]
Alkali-cation affinities of polyoxyethylene dodecylethers and helical conformations of their cationized molecules studied by electrospray mass spectrometry]
DOI:10.1016/j.jasms.2007.08.004 URL [Cited within: 1]
Complex formation via hydrogen bonding between Rhodamine B and montmorillonite in aqueous solution
DOI:10.1038/s41598-017-18057-8 URL [Cited within: 1]
Hybrid organo-inorganic clay with nonionic-interlayers. Mid- and near-IR spectroscopic studies
DOI:10.1021/jp200845e URL [Cited within: 1]
A thermodynamic understanding of clay-swelling inhibition by potassium ions
DOI:10.1002/(ISSN)1521-3757 URL [Cited within: 1]
Mechanism of adsorption and desorption of water vapor by homoionic montmorillonites: 2. The Li (super +), Na (super +), K (super +), Rb (super +), and Cs (super +)-exchanged forms
DOI:10.1346/CCMN URL [Cited within: 1]
Effect of layer charge on the intercalation of poly(ethylene oxide) in layered silicates: Implications on nanocomposite polymer electrolytes
DOI:10.1021/cm990677p URL [Cited within: 1]