Introduction
Shale gas is an important replacement resource for conventional oil and gas that affects the strategic energy plans of many countries. The development of shale gas is receiving increasing attention[1-2]. Shale reservoirs are composed of fine-grained sediment rich in clay minerals and organic matter, and have massive micro/nanopores and strong heterogeneity. Imbibition driven by capillary force is an important mechanism to improve the production of shale gas wells[3-4]. During the fracturing process, fracturing fluid intruding into the formation will lead to increase of water saturation in the near well area, hence increase in capillary resistance at the gas-water interface and the Jarmin effect, and flow difficulty of fracturing fluid, then causing water blocking[5]. Water block is a transient phenomenon. Shale reservoir is characterized by high imbibition ability, which improves the diffusion of fracturing fluid. Under the effect of capillary force in hydrophilic small pores, the fracturing fluid originally trapped in the main flow passages of shale gas will be absorbed into the matrix pores, making the flow channels gradually open, namely, the water lock is removed[6]. Zhang et al.[7] used the Boltzmann model to simulate gas flooding in micro/nanopores and found that after forming a continuous phase in large pores, gas was difficult to displace water in small pores, resulting in water blocking damage and impeding shale gas production. Cheng et al.[8] believed that the countercurrent imbibition of fracturing fluid into the matrix can displace the gas. Therefore, the micro-nano pore structure of shale with certain imbibition and diffusion ability dictates its water block removal potential. The water absorption characteristic of shale is related to the number, size, volume, and pore-fracture connectivity of micro-nanopores[9].
When the fracturing fluid immerses into the shale reservoir, hydration occurs, and the shale microstructure changes. At present, there is no consensus on whether hydration has favorable effect on the physical parameters of shale. Sui et al.[10] compared the microscopic pore structure variations before and after hydration through scanning electron microscopy observation and found that hydration contributed to the formation of dissolution pores and fall-off of mineral grains, larger pore size, and microfractures propagate and interconnect in the shale. Hower[11]pointed out that after fracturing fluid infiltrated into shale, the formation fluid would change in ion concentration, clay minerals would swell, and fine particles would disperse and migrate, which causes pore blockage. Farah et al.[12] believed that the pores in shale with small sizes had large capillary force, and clay minerals would swell after hydration, blocking pore-throats, and thus making pore connectivity poor. In conclusion, shale hydration can be made use to induce microfractures to form complex fracture network connecting micro/nanopores[4]. However, imbibition may cause water blocking damage and reduce gas permeability in shale. Therefore, it is of great significance to study the influence of hydration on pore structures and the change in physical properties of shale after fracturing fluid invasion to optimize fracturing parameters and improve the stimulation effect of shale reservoirs.
Shale samples from the Longmaxi Formation in the Sichuan Basin, which are rich in organic matter and developed with micro/nanopores, were selected. Various approaches, including scanning electron microscopy (SEM), CT scanning techniques, high-pressure mercury injection, low-temperature nitrogen adsorption method, and imbibition experiments, were utilized to compare the hydration characteristics of montmorillonite and illite, reveal the evolution mechanism of the pore structure during hydration from micro to macro perspectives, analyze the major factors affecting water adsorption and water block removal ability of shale, and reveal the corresponding relationship between the pore structure and water block removal ability. This study can provide basis for the optimization of fracturing fluid formula and soaking time.
1. Hydration experiments of clay minerals
Clay mineral crystals are composed of layered silica-aluminate with water in surface contact. The surface of the unit crystal layer is negatively charged, which can absorb hydrated cations and form a hydration film. Fracturing fluid infiltrating into matrix pores changes the ion types and ion concentration distribution of the original pore fluid, and further affects the ion types and ion concentration distribution on the surface and interlayer of clay minerals, which leads to a series of physical and chemical reactions. Therefore, the core of shale hydration is clay mineral hydration[13], and hydration strain and hydration stress are the key parameters to evaluate hydration intensity.
Hydration characteristics are different for different clay mineral species. Chlorite is oil wet, and its crystal structure is relatively stable. Kaolinite has weak hydrophilicity. Both of them don’t swell when contacting with water[14]. In contrast, the hydration of montmorillonite and illite is strong, so this work focused on hydration characteristics of them.
1.1. Samples and methodology
Samples: Illite powder and montmorillonite powder (0.075 mm, 200 mesh) were selected. After purification by extraction method, 4 g of each sample was taken and put into a cylindrical sample tube.
Process: (1) An external pressure of 1.5 MPa was applied to the sample tube for compaction, and the initial height of the sample was recorded after the pressure was applied for 10 min. (2) The sample was fixed in a bucket of NP-3 clay dilatometer, and then deionized water, CaCl2 solution of 1.0 mol/L, NaCl solution, and KCl solution were added. (3) The heights of the samples at different times soaked at room temperature and pressure were recorded. (4) A certain external pressure was applied to the sample to ensure a constant volume and measure the hydration stress.
1.2. Results
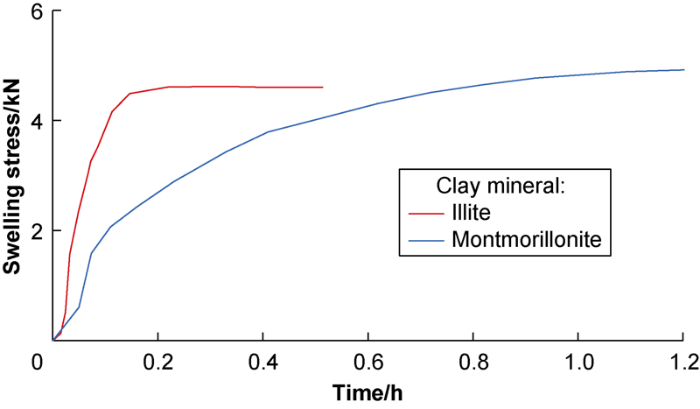
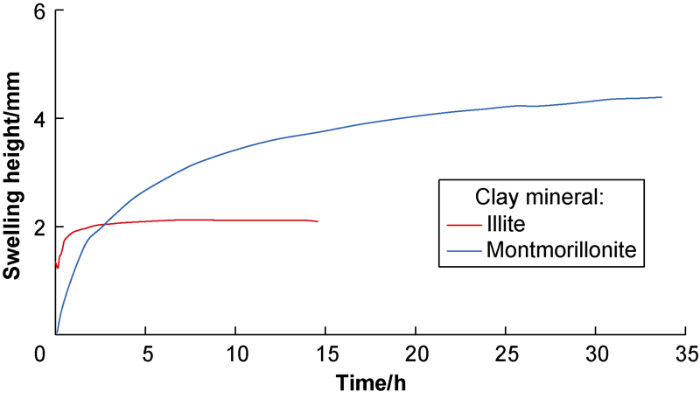
The hydration stress and swelling height curves of illite and montmorillonite in deionized water are shown in Figs. 1 and 2, respectively. From the results, (1) The maximum hydration expansion stress of illite is slightly higher than that of montmorillonite, but the increase rate of hydration stress of illite is much higher than that of montmorillonite and basically constant, and reaches equilibrium at about 0.17 h. The hydration stress of montmorillonite increases slowly, and reaches equilibrium at 1.0 h. (2) When the hydration stress reaches equilibrium state, the volume continues to increase; that is, the hydration strain change lags behind that of the hydration stress. (3) The hydration swelling capacity of montmorillonite is much higher than that of illite but slower. (4) Illite has smaller swelling volume but faster hydration rate, and reaches equilibrium in a short time. The illite has faster and more intense hydration with higher intensity at particle surface.
Fig. 1.
Fig. 1.
Hydration stress curves of montmorillonite and illite in deionized water.
Fig. 2.
Fig. 2.
Swelling height curves of montmorillonite and illite in deionized water.
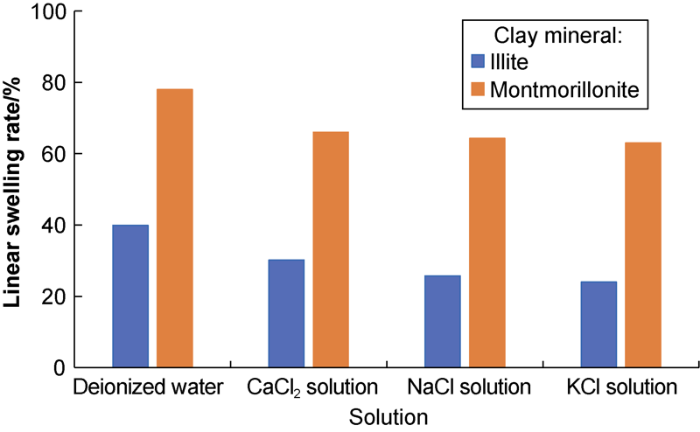
The linear swelling rates of montmorillonite and illite in the solutions are shown in Fig. 3. The figure shows that they have smaller linear swelling rates in inorganic salt solution than in deionized water, indicating that inorganic cations can inhibit the hydration swelling of clay minerals, the inhibition effect on illite is stronger, and K+ has the best inhibition effect.
Fig. 3.
Fig. 3.
Linear swelling ratios of montmorillonite and illite in different solutions.
The hydration strain change of clay minerals lags behind that of hydration stress. Hydration has both positive and negative effects. On the one hand, the hydration stress acts as a tensile force on mineral particles[14], while the hydration stress compresses the surrounding nonswelling parts, which will reduce the rock cementation strength, induce particle disintegration and dispersion, and thus make tensile fractures extend and connectivity of pores and fracture increase. With the increasing hydration time, the hydration stress reaches the equilibrium state, but the hydration strain continues to increase. When the tensile stress generated by hydration stress on the microfracture surface is not sufficient to support continuous expansion, the swelling of clay minerals will occupy part of the pore volume and reduce the fracture size and connectivity between pores and fractures[15].
2. Characterization of microstructure of shale sample before and after hydration
The hydration characteristics of montmorillonite and illite are quite different and have different effects on the changing ability of the shale hydration microstructure and the diffusion ability of fracturing fluid. In this paper, the changes in pore structure, such as particle morphology and pore size distribution, during the hydration process of two sets of shale samples with different clay mineral compositions were compared through SEM, micro-CT scanning, high pressure mercury injection, low temperature N2 adsorption and imbibition experiments, and the main controlling factors affecting shale hydration and water block removal ability and the effects of clay minerals on micro-structure of hydrated shale were analyzed.
2.1. Experiment design
Samples: The shale samples were collected from two adjacent well areas of the Lower Silurian Longmaxi Formation in the Changning area, Sichuan Province, and the samples were numbered L1 and L2. The measured TOC and XRD mineralogy results are shown in Table 1. The mineral composition was mainly quartz and clay minerals. The two samples have similar total amount of clay minerals, but differ widely in the composition of clay minerals. L1 is dominated by illite, with a content of 22.1%. L2 is dominated by montmorillonite, with a content of 23.3%.
Table 1. TOC and mineral composition of L1 and L2 shale samples.
| Sample No. | TOC/% | Proportion of non-clay mineral/% | Proportion of clay minerals/% | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Quartz | Orthoclase | Plagioclase | Pyrite | Dolomite | Calcite | Illite | Chlorite | Montmorillonite | ||
| L1 | 2.3 | 38.5 | 3.9 | 4.3 | 2.8 | 2.3 | 6.5 | 22.1 | 8.4 | 8.9 |
| L2 | 3.5 | 36.1 | 3.5 | 4.7 | 2.9 | 3.4 | 4.5 | 11.2 | 6.9 | 23.3 |
Equipment: (1) A Quanta450 environmental scanning electron microscope was used to observe and analyze the microstructure of the shale samples. It has a resolution in the secondary electron high vacuum mode of 3-8 nm and backscattered electron resolution of 4 nm, and can measure the type, size, number of pores, and development of microfractures[16]. (2) A MicroXCT-400 CT machine with an X-ray spatial resolution up to 1.5 μm was used for rock face scanning. (3) A Poremaster 60GT mercury injection apparatus, with a maximum mercury injection pressure of 413.7 MPa and an optimal sample pore size range of 20-1500 nm, was used to do the high pressure mercury injection experiment. (4) An automatic pore measurement system was used for low temperature N2 adsorption experiment. (5) A Sartorius QUINTIX224-1CN analytical balance with a measuring range of 220 g and an accuracy of ±0.000 1 g was used for the hydration imbibition experiments.
Experimental process: (1) Multiple standard core plugs (2.54 cm in diameter and 5.00 cm in length) were drilled from adjacent positions of two sets of full diameter cores (L1 and L2). Cube shale samples with 1 cm side lengths were taken from the adjacent positions of the same end face of L1 and L2 standard core plugs respectively for scanning electron microscopy. The standard core plugs were polished into cylinders with a diameter of 2.50 cm and a height of 2.83 cm for micro-CT scan, and cylinders with 1.00 cm in height and 1.00 cm in diameter for high-pressure mercury injection experiment. Then, some standard core plugs were grounded into granular samples with particle sizes of 0.250-0.425 mm (40-60 mesh) for nitrogen adsorption experiment. (2) Since the same rock sample cannot be soaked for four times, so four portions of each sample in the two sets of shale samples (L1 and L2) in four sizes were soaked for 0, 5, 10, and 20 d respectively at 90 °C. The same fracturing fluid formula was used on site: 0.1% resistance reducing agent+0.1% anti-swelling agent+1% drainage agent. (3) After argon ion polishing and carbon film spraying, the surfaces of the cubic samples were dried at 60 °C, and the microstructures of samples were observed by scanning electron microscopy. (4) Vertical fractures were cut in the core plugs without hydration along the diameter direction to simulate fracturing fractures. A CT scanning machine was used to scan along the end face of the core plug. Then, the core column was placed in the holder of the displacement device, and the experimental fracturing fluid was injected to simulate the fracturing process of the underground shale. The displacement pressure was set at 7.5 MPa, and the displacement flow gradually decreased from 2.0 mL/min to 0.2 mL/min. After 10 d of displacement, the samples were scanned along the end face to study the hydration microfractures and pore connectivity inside. (5) The Poremaster 60GT mercury injection apparatus was taken to measure the pore size distribution. (6) The granular shale samples were degassed at 150 °C for 5 h, and then the isothermal adsorption and desorption curves of them were measured and calculated by the static volume method at 77 K. (7) The cubic shale samples with the side length of 1 cm were placed in the oven for drying for 10 h and then put into the fracturing fluid to monitor the changes of the sample mass over time, and the sample mass was recorded once every 2 min.
2.2. Analysis of experimental results
2.2.1. Changes in particle surface morphology of shale end face
Figs. 4 and 5 show the changes in the microstructure of shale samples L1 and L2 over time. The scanning electron microscopy (SEM) images show that before the shale samples were soaked, the pores and microfractures in clay layers were relatively developed, with the morphology of a narrow slit, which was partially opened but not connected. The initial microfractures of L2 shale samples were more developed (Figs. 4a and 5a).
Fig. 4.
Fig. 4.
Microstructure images of L1 shale sample at different soaking times.
Fig. 5.
Fig. 5.
Microstructure images of L2 shale sample at different soaking times.
There are three types of changes in the hydration process of the L1 sample: (1) After being soaked, the sample had no obvious swelling of clay minerals, but mainly opening of bedding cracks and growth of initial microfractures, and the microfractures induced by hydration were mainly the opening and connectivity of fractures between clay laminae parallel with the bedding direction (Fig. 4b). (2) As the clay minerals hydrated and swelled, the edge of the particles passivated gradually; and the clay enrichment area had a higher expansion potential. Under the compression of the larger swelling stress, the surrounding nonexpanding particles disintegrated, filling in the pores, and the microfractures tended to close (Fig. 4c). (3) A large number of particles and banded polymers adhered to the rock surface, and with increase of dissolved pores, the shale sample turned porous and loose in overall structure, resulting in a decrease of cementation strength of shale samples (Fig. 4d). The variation trend of the L2 shale samples is similar to that of L1 (Fig. 5). The difference is that distinct fracture propagation behavior is still observed in L2 shale samples after hydration for 10 d, and the dispersion degree of particles is much higher than that of L1 samples after hydration for 20 d.
During hydration, the microstructural evolution characteristics of the L1 and L2 samples are quite different. The L1 shale sample had mainly development of hydration-induced microcracks, while L2 had a higher degree of clay particle expansion and dispersion. Comparison of Figs. 4 and 5 shows that the shale with higher montmorillonite content has stronger dispersion of particles and is more likely to hydrate and swell. In contrast, the shale sample with higher illite content has lower dispersion of particles, and isn’t susceptible to hydration and expansion, and mainly has mainly microcrack expansion[10].
2.2.2. Distribution characteristics of pores and microfractures in end faces of the shale samples
The microfractures of L2 shale samples extend significantlyafter hydration for 10 d. Therefore, a micro-CT scan were performed on the end faces of L2 samples after drying and displacement for 10 d respectively to observe the plane distribution characteristics of microfractures and pores (Fig. 6). It can be seen from the figure that: (1) The shale sample had one artificial fracture and distinct bedding fractures (No. 1 and No. 2 areas). Under hydration, the No. 1 and No. 3 areas had distinct fracture extension, resulting in stronger connectivity of microfrac-tures. (2) The No. 2 area changed from green to red, indicating substantial increase of the bedding fracture volume. (3) Small-scale scattered micro-fractures (the blue discrete points) around the artificial fracture increased in number considerably.
Fig. 6.
Fig. 6.
Microstructure images of L2 shale samples after drying and displacement for 10 d.
In conclusion, the shale sample before hydration had scattered small-scale fractures. After hydration, the clay swelled and microfractures opened, and small-scale pore fractures were densely distributed and interconnected in local parts, enhancing the connectivity between fractures, organic pores, and inorganic pores, and increasing the reservoir permeability, which is conducive to the diffusion of fracturing fluid[17].
2.2.3. Pore size distribution after imbibition
2.2.3.1. High-pressure mercury injection experiment
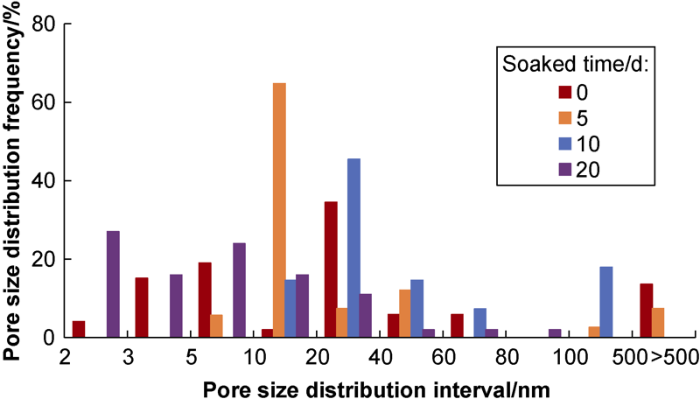
The percentages of pores in different sizes of L1 and L2 samples before and after hydration calculated according to the mercury injection pressure curves are shown in Figs. 7 and 8. For the L1 shale sample, compared with the unhydrated shale sample, the pores of 2-10 nm disappeared, the pores of 10-20 nm increased by 1.25 times, the pores of 20-40 nm decreased slightly, and pores over 100 nm began to appear after hydration for 5 d. Compared with the situation of hydration for 5 d, the sample after 10 d of soaking had none of pores of 2-10 nm still, significant decrease of pores of 10-20 nm, significant increase of pores of 20-40 nm, and slight increase of pores larger than 100 nm. Compared with the situation of hydration for 10 d, the sample after 20 d of soaking had pores of 2-10 nm approximately returning to the state before soaking, slight increase of pores of 10-20 nm, and significant reduction of pores of 20-40 nm and over 100 nm.
Fig. 7.
Fig. 7.
Comparison of pore size distribution of the L1 shale sample before and after being soaked.
Fig. 8.
Fig. 8.
Changes in pore size distribution of the L2 shale sample before and after being soaked.
For the L2 shale sample, compared with the situation before being soaked, the sample after being soaked for 5 d had almost none of pores of 2-10 nm, increase of pores of 10-20 nm by 31.4 times, decrease of pores of 20-40 nm by 78.5%, and pores over 100 nm remained almost the same. Compared with the situation after hydration for 5 d, the sample after 10 d of soaking had none of pores of 2-10 nm still, significant decrease of pores of 10-20 nm, significant increase of pores of 20-40 nm, and slight increase of pores larger than 100 nm. Compared with the situation after being soaked for 10 days, the sample after 20 d of soaking had pores of 2-10 nm proximately returning to the state before soaking, slight increase of pores of 10-20 nm, significant decrease of pores of 20-40 nm, and none of pores larger than 100 nm.
It can be seen that appropriate soaking can improve the pore structure of shale samples and enhance the flow capability of shale gas. But it is not the longer the hydration time, the better. After being soaked for 5 d, the samples had substantial increase of pores of 10-20 nm and opening of microfractures larger than 100 nm. After being soaked for 10 d, the samples had significant increase of pores of 20-40 nm and microfractures of more than 100 nm remaining open. After being soaked for 20 d, the samples had most of 10-40 nm pores blocked and microfractures larger than 100 nm approximately closed. In short, the L1 shale sample after being soaked for 5 d and L2 shale sample after being soaked for 10 d had most developed pore structure and highest permeability.
2.2.3.2. Low temperature nitrogen adsorption experiment
There is a hysteresis loop between nitrogen adsorption and desorption isotherms, and the hysteresis loop widens at higher relative pressures (p/p0>0.45). The shape of the pores can be determined according to the shape of the hysteresis loop[16]. Boer et al.[18] proposed that the hysteresis loops can be divided into five types (types A-E). On this basis, the IUPAC (International Union of Pure and Applied Chemistry) recommended four types (H1- H4)[19], which represent cylinder pore, ink-bottle-shaped pore, slit-shaped pore, and wedge-shaped pore with one or two ends opened respectively.
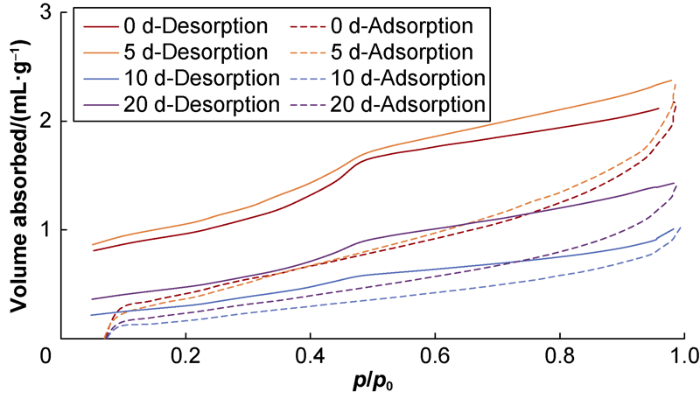
Figs. 9 and 10 show the nitrogen adsorption and desorption curves of L1 and L2 samples. It can be seen the hysteresis loop of L1 shale sample before being soaked is close to H2 type and has some features of H3 type, so the pores in the sample then consisted of ink-bottle-shaped and wedge-shaped ones. Compared with the situation before being soaked, the shale sample after being soaked for 5 d had steeper desorption curve and wider hysteresis loop in the medium pressure range (0.45-0.80), which was similar to the Boer B type but also had the characteristics of the H4 type, indicating that the pores had the characteristics of slit-shaped pores. Compared with the situation after 5 d of soaking, the sample after being soaked for 10 d had narrower hysteretic loop in the range of high pressure (0.8≤p/p0<1.0) and characteristics of H3 type, indicating that the pores changed into wedge-shaped again. Compared with the situation after being soaked for 10 d, the sample after being soaked for 20 d had wider hysteretic loop and more distinct H2 characteristics.
Fig. 9.
Fig. 9.
N2 isothermal adsorption and desorption curves of the L1 shale sample.
Fig. 10.
Fig. 10.
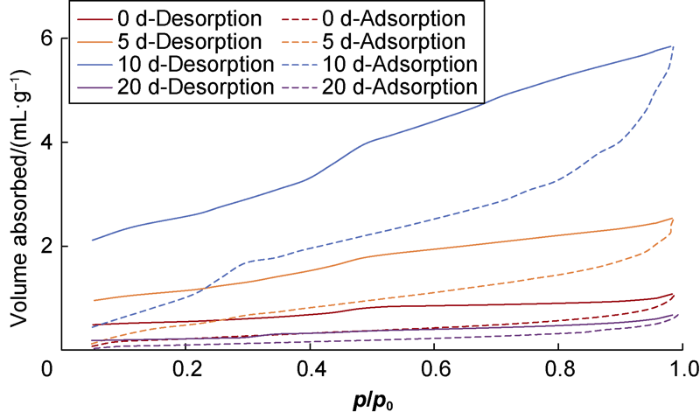
N2isothermal adsorption and desorption curves of the L2 shale sample.
During the soaking from 0 to 5 d then to 10 d, the L2 shale sample showed similar evolution trend in hysteresis loop, that is, the hysteresis loop gradually changed from H2 type to Boer B type, with B type characteristics becoming more and more distinct. After being soaked for 20 d, the sample had very narrow hysteresis loop, indicating it had largely closed pores.
It can be seen that during soaking, the hysteresis loop of this sample first changed from H2 type to Boer B type, and then to H3 type, indicating that the pores turned from ink-bottle-shape to slit-shaped, and then to wedge-shaped, namely, the degree of pore opening on the whole increased first and then decreased. This means that shale has optimal hydration time when the pore structure is most developed and the physical properties are the best. Hydration time too long would have negative effect. The experimental results show that the L1 shale sample soaked for 5 d and the L2 shale sample soaked for 10 d had the best connectivity of pores and fractures and the strongest diffusion capacity of fracturing fluid.
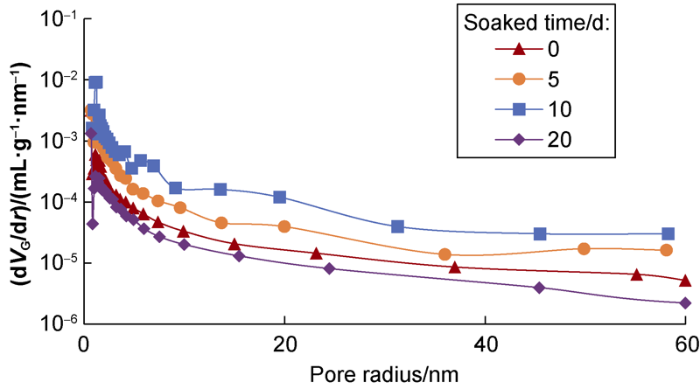
Based on data from Figs. 9 and 10 and the BJH model[20], the differential of the pore volume per unit weight to the pore radius was used to reflect the changing trend of the pore size distribution to eliminate the influence of the sampling interval (Figs. 11 and 12).
Fig. 11.
Fig. 11.
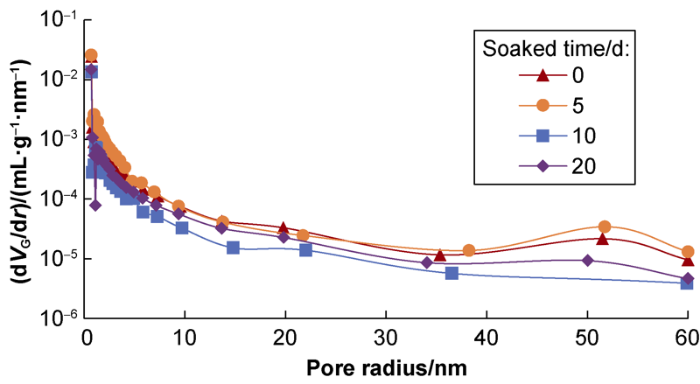
Variations of pore size distribution of the L1 shale sample.
Fig. 12.
Fig. 12.
Variations of pore size distribution of the L2 shale sample.
The four soaking curves of L1 shale sample show that the curve after being soaked for 5 d is roughly at the highest level, indicating that the pore volume of the sample under this condition is the largest, while the curves of the sample after being soaked for 10 d and 20 d are lower than the curve of the sample before being soaked. Similarly, the curves of the L2 shale sample after being soaked for 5 d and 10 d are higher than the curve of the sample before being soaked, and the curve of the sample after being soaked for 10 d is highest, indicating the sample has the largest pore volume under this condition. It follows that proper soaking can improve the physical properties of reservoir, but soaking can cause damage to reservoir when time is too long.
2.2.4. Water imbibition experiment
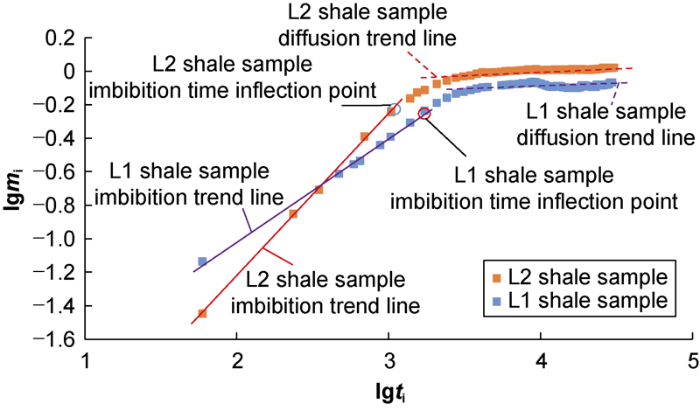
The imbibition experiment is useful to analyze the migration of fracturing fluid in shale. Based on the study of Hu et al.[21], the log-log imbibition curve usually has at least two straight sections: the initial straight line section is called the imbibition section, the final straight line section is called the diffusion section, and the end time of the imbibition section is the inflection point. To characterize the diffusion capacity of fracturing fluid in pore network, the diffusion capacity is defined as the ratio of the imbibition mass in the diffusion section to the final imbibition mass[21]. The imbibition curves of the samples were plotted in a double logarithmic coordinate system (Fig. 13). It can be seen that the L1 shale sample enters the diffusion stage after imbibition for 1720 min, and its diffusion capacity is 0.36. In contrast, the L2 shale sample enters the diffusion section after imbibition for 1040 min, and its diffusion capacity is 0.47, 1.32 times that of the L1 shale sample.
Fig. 13.
Fig. 13.
Curves of imbibition mass over time of L1 and L2 shale samples in a double logarithmic coordinate system.
The imbibition curve can be roughly divided into three stages: rapid rise, slow rise, and stabilization. In the rapid rise stage (imbibition time of less than 1720 min for the L1 shale sample and imbibition time of less than 1040 min for the L2 sample), under the combined action of osmotic pressure difference and capillary force, fracturing fluid imbibes into inorganic pores and micro-fractures in priority, water adsorption by micro-fractures and inorganic pores take dominance[22]. In the slow rise stage (the imbibition time of approximately 1720-3450 min for the L1 shale sample and 1040-3790 min for the L2 shale sample), fracturing fluid diffuses into deep matrix pores, the flow resistance increases, the water absorbed by micro-fractures and inorganic pores decreases, and the water absorption is mainly by clay minerals[23-24]. In the stabilization stage (the imbibition time of greater than 3450 min for the L1 shale sample and greater than 3790 min for the L2 shale sample), the imbibition mass of the L1 shale sample fluctuates at approximately 0.83 g and that of the L2 shale sample at approximately 1.03 g, indicating that the shale sample is saturated with water.
It can be seen from Figs. 7 and 8 that the L1 sample has a higher proportion of pores smaller than 20 nm, smaller pore sizes and stronger capillary force, so it has stronger imbibition in the initial stage but insufficient diffusion capacity, and water molecules are likely to block pore passages, causing water block. In contrast, the L2 shale sample has more pores greater than 20 nm in diameter and larger overall pore size (Fig. 8), so it has weaker imbibition but stronger diffusion ability in the initial stage. Therefore, the L2 shale sample has certain ability to remove water block by itself.
In conclusion, different clay minerals have different hydration stress and strain characteristics. With mainly electric double layer repulsion as interlayer force, illite only has surface hydration[13], thus is higher in hydration stress, smaller in hydration strain, and shorter in hydration equilibrium time[25]. In contrast, the hydration of montmorillonite is driven by osmotic pressured difference between fracturing fluid and pore fluid, so after surface hydration, montmorillonite will have large swelling range dozens times of the layer spacing, so it features small hydration stress, large hydration strain, and long hydration equilibrium time[25]. It follows that shale layers with different clay mineral compositions differ somewhat in hydration characteristics macroscopically. Therefore, the L2 sample with higher montmorillonite content is prone to hydration swelling, higher in uneven expansion degree, and has more significant changes in microscopic pore structure and higher potential in reservoir physical property improvement; while the L1 shale sample with higher illite content does not easily hydrate and swell, and has lower dispersibility, mainly microfracture expansion, and limited pore structure improvement caused by hydration.
The variation pattern of shale microstructure caused by hydration has a time effect, and the variation degree is related to the composition of clay minerals. Hydration controls gas flow in shale by affecting pore structure of shale. Clay hydration can enhance the connectivity between micro/nano-organic pores and inorganic pores and expand the pore space, which can improve the flow capacity of shale gas. After fracturing, the reservoir physical properties can be improved to a certain extent by shutting down the well for soaking for proper time.
3. Field application
For the Longmaxi Formation shale (L1) with higher illite content in well area A located in the Changning area, Sichuan Basin, the recommended optimal soaking time is 5 d. For the shale (L2) in well area B with higher montmorillonite content, the recommended optimal soaking time is 10 d. Two adjacent wells in each well area, namely, Well A-1 and Well A-2 in well area A and Well B-1 and Well B-2 in well area B, were selected for field test. The two wells in the same well area have similar physical properties, fracturing parameters, and mineral composition (Table 2). Well A-1 was soaked for 5 d, and Well A-2 was soaked for 10 d. Well B-1 was soaked for 10 d, and Well B-2 was soaked for 15 d.
Table 2. Comparison of the effect of different soaking times on reservoir stimulation.
| Well Area | Well No. | Vertical Depth/m | Horizontal section length/m | Stage number | Montmorillonite content/% | Illite content/% | Optimal soaking time/d | Field soaking time/d | Production/(104 m3•d-1) |
|---|---|---|---|---|---|---|---|---|---|
| A | A-1 | 2590 | 1478 | 22 | 7.4 | 18.3 | 5 | 5 | 21.1 |
| A-2 | 2620 | 1505 | 23 | 5.6 | 20.1 | 5 | 10 | 21.2 | |
| B | B-1 | 2780 | 1400 | 29 | 22.1 | 9.2 | 10 | 10 | 40.1 |
| B-2 | 2750 | 1500 | 34 | 20.4 | 10.4 | 10 | 15 | 29.7 |
The test results show that Well A-1 shut in for 5 d according to the optimal soak time, while Well A-2 with longer soaking time of 10 d. The number of fracture stages in Well A-1 was less than that in Well A-2, but the measured production was roughly the same. Compared with Well B-1, although Well B-2 has more fracturing stages and longer horizontal section, it had a production 25.9% lower than Well B-1 as its soaking time is longer than the optimal time. This indicates that the wells with higher content of montmorillonite have a longer optimal soaking time; wells with higher illite content have a short optimal soaking time. A shut-in for optimal soaking time is helpful to improve the stimulation effect of shale reservoirs. Extending the soaking time will damage the physical properties of shale reservoirs.
4. Conclusions
The hydration characteristics of shale are closely related to the composition of clay minerals. Shale with higher illite content has low dispersion, is less liable to hydrate and swell, dominated by microfracture expansion, and has a limited ability to improve the pore structure. The shale with higher content of montmorillonite leads to strong dispersion, is easier to hydration and expansion, and has higher potential of microscopic pore structure improvement by hydration.
Shale with higher illite content has a higher proportion of small pores, higher capillary force, and relatively stronger initial imbibition, but lower diffusion ability, so water molecules can block pore passages, forming water block. In contrast, shale with higher content of montmorillonite has a high proportion of large pores, and relatively weaker initial imbibition. In this kind of shale, after entering large pore channels, fracturing fluid will be imbibed to medium-small pores, resulting in good diffusion ability, so the shale reservoir of this kind has certain ability to remove water block by itself.
Shale reservoir has an optimal hydration time when it has the most developed microscopic pore structure and the best physical properties. Hydration time too long would damage physical properties of shale reservoirs. The optimal hydration time of shale with a high illite content is shorter than that of shale with high montmorillonite content.
Inorganic cations can inhibit hydration swelling of clay minerals and have stronger inhibition to illite, and K+ has the best inhibition to clay hydration and swell. For reservoirs with a high content of montmorillonite, the cation content of fracturing fluid can be reduced to promote clay hydration. For illite-rich reservoirs, fracturing fluid with high K+ content can be injected to suppress hydration.
Nomenclature
mi—Imbibition mass, g;
p—pressure during low temperature N2 adsorption, MPa;
p0—saturation vapor pressure, MPa;
r—pore radius, nm;
ti—imbibition time, min;
VG—pore volume per unit sample weight, mL/g.
Reference
Shale oil and gas revolution and its impact
A transient productivity model of fractured wells in shale reservoirs based on the succession pseudo-steady state method
DOI:10.3390/en11092335 URL [Cited within: 1]
Prediction of oil recovery in naturally fractured reservoirs subjected to reinfiltration during gravity drainage using a new scaling equation
Optimized completion design for triggering a fracture network to enhance horizontal shale well production
Evaluation of gas wettability and its effects on fluid distribution and fluid flow in porous media
DOI:10.1007/s12182-013-0303-4 URL [Cited within: 1]
Imbibition and water blockage in unconventional reservoirs: Well-management implications during flowback and early production
DOI:10.2118/167698-PA URL [Cited within: 1]
The explanation of low recovery of fracturing fluid in shale hydraulic fracturing by pore-scale simulation
Impact of water dynamics in fractures on the performance of hydraulically fractured wells in gas shale reservoirs
DOI:10.2118/127863-PA URL [Cited within: 1]
Analytical spontaneous imbibition model for confined nanofractures
Investigation of microscopic pore structure variations of shale due to hydration effects through SEM fixed-point observation experiments
Simulation of the impact of fracturing-fluid-induced formation damage in shale gas reservoirs
DOI:10.2118/173264-PA URL [Cited within: 1]
Quantitative characterization of micro forces in shale hydration and field applications
Evaluation of impact of clay mineral fabrics on hydration process
The fate of fracturing water: A field and simulation study
DOI:10.1016/j.fuel.2015.09.040 URL [Cited within: 1]
Full-scale pores and micro-fractures characterization using FE-SEM, gas adsorption, nano-CT and micro-CT: A case study of the Silurian Longmaxi Formation shale in the Fuling area, Sichuan Basin, China
DOI:10.1016/j.fuel.2019.04.116 URL [Cited within: 2]
Prediction of shale apparent liquid permeability based on fractal theory
DOI:10.1021/acs.energyfuels.0c00318 URL [Cited within: 1]
Studies on pore systems in catalysts: VII. Description of the pore dimensions of carbon blacks by the t method
DOI:10.1016/0021-9517(65)90264-2 URL [Cited within: 1]
Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity
DOI:10.1351/pac198254112201 URL [Cited within: 1]
Characterization of mechanochemically synthesized MOFs
DOI:10.1016/j.micromeso.2011.11.039 URL [Cited within: 1]
Low pore connectivity in natural rock
DOI:10.1016/j.jconhyd.2012.03.006 URL [Cited within: 2]
New pore space characterization method of shale matrix formation by considering organic and inorganic pores
DOI:10.1016/j.jngse.2015.08.017 URL [Cited within: 1]
A unified multiple transport mechanism model for gas through shale pores
Gas mass transport model for microfractures considering the dynamic variation of width in shale reservoirs
DOI:10.2118/194494-PA URL [Cited within: 1]
Mechanisms of water adsorption into partially saturated fractured shales: An experimental study
DOI:10.1016/j.fuel.2015.07.015 URL [Cited within: 2]