Introduction
Oil-based drilling fluids have obvious edge in lubricating, inhibiting shale hydration expansion, inhibiting formation slurry formation, stabilizing wellbore, and resisting high temperature and salt-calcium pollution. They are especially suitable for drilling in complex formation environment and unconventional oil and gas reservoirs such as shale gas reservoir[1,2,3,4]. However, conventional oil-based drilling fluids are hard to wash, leading to difficulties in well completion and cementing, difficult disposal of oily cuttings, and serious environmental pollution. These issues seriously hinder the promotion and application of oil-based drilling fluids[4,5,6,7].
Since the 1980s, relevant studies have shown that surfactants such as emulsifiers in oil-based drilling fluids are double-edged. They endow the oil-based drilling fluids with high emulsifying stability and strong oil-wettability which are the necessary conditions for the excellent drilling performance of the drilling fluid, but also cause difficult well washing and environmental pollution[8,9,10,11,12,13,14,15,16].
Stimuli-responsive emulsifiers were proposed to replace the traditional emulsifiers to make the oil-based drilling fluids reversible. This way allows the oil-based drilling fluid to remain the advantages in drilling performance while control its adverse effects on oil and gas production capacity and ecological environment from the source. In other words, the oil-based drilling fluid exists in the form of oil-based drilling fluid and gives full play of its edges in drilling, while in the completion stage after drilling, it can be reversed to water-based drilling fluid by external stimulus induction, to make the drilling fluid easy to be cleaned[17,18,19,20].
The present methods of inducing the oil-based drilling fluid to reverse commonly use acids (e.g., hydrochloric acid and acetic acid)[21-24] or polyvalent metallic salts[25]. But acid and polyvalent metal salts added would accumulate in the drilling fluid, affect the drilling fluid performance, and cause secondary pollution[26,27,28]. In view of this, it is necessary to find greener stimulation methods. In addition, the previous studies on the reversibility of oil-based drilling fluid aimed to enhance the efficiency of oily cutting treatment and filter-cake cleanup without considering the application of the reversibility in controlling the rheological behaviors and solid content.
In this work, CO2 and CaO green and low in cost were used to stimulate the amine emulsions with tertiary amine non-ionic surfactants as emulsifiers. The responsive behaviors (i.e., emulsion inversion and rheological transition) of the emulsion system under the stimulus of CO2/CaO and mechanisms were examined, and the application characteristics of the CO2/CaO responsive behaviors in oil-based drilling fluids were investigated via filter-cake and oily cuttings cleanup test, control of low density useless solids test and oil-based drilling fluid recycling tests.
1. Formula and CO2/CaO responsive behaviors of emulsion
1.1. Emulsion formula
An oil-based drilling fluid is a colloidal dispersion system based on emulsion. The emulsion used is not only directly related to the drilling performance of the fluid, but also the material basis of CO2/CaO response behaviors of the fluid and determines the unique cleaning, solid control and recycling performances of the fluid.
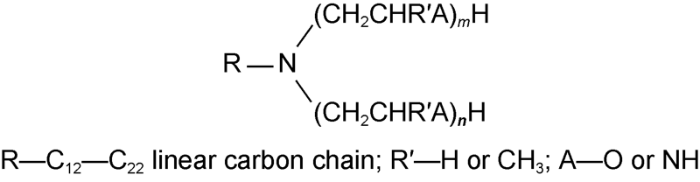
Several initial amine emulsions were prepared via blending 3# white oil, tertiary amine non-ionic surfactant, and 25 w/v% CaCl2 brine at 8000 r/min and room temperature for 40min. The oil/water volume ratios were 50:50, 60:40 and 70:30. The tertiary amine non-ionic surfactant had a mass fraction of 5% in the emulsion and a hydrophile-lipophile balance (HLB) of 5-6. The chemical structural formula of the tertiary amine non-ionic surfactant is shown in Fig. 1.
Fig. 1.
Fig. 1.
Chemical structural formula of tertiary amine non-ionic surfactant.
1.2. Carbon dioxide/calcium oxide responsive behaviors
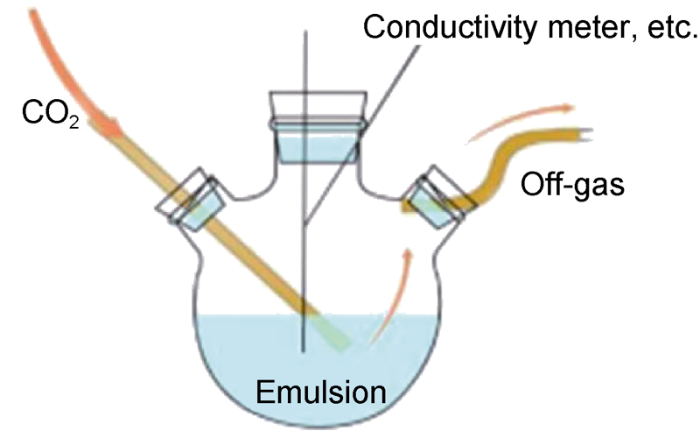
The CO2/CaO responsive behaviors of the prepared amine emulsion are mainly manifested in two aspects: emulsion inversion and rheological transition. In order to analyze the response behaviors of the amine emulsion, the following experiments were carried out: (1) 100 mL of emulsion was added into the bubbling experimental device (Fig. 2). CO2 was injected into the device at a flow rate of 0.15 L/min under continuous low-speed stirring of 300 r/min. (2) During CO2 injection, changes in conductivity, emulsion breaking voltage, pH value of the emulsion were measured using conductivity meter, emulsion breaking voltage meter, precision pH test paper respectively, and water solubility of the emulsion was observed to find out the emulsion inversion characteristics of the amine emulsion under CO2 induction. (3) Emulsions at different CO2 injection times were taken, and their viscosities as function of shear rate were tested on a HAAKE MARSIII rotary rheometer to find out the rheological transition characteristics of the amine emulsion under CO2 induction. For each measurement, the emulsion sample was vigorously stirred for one minute, and the relevant tests were then run at decreasing shear rates from 1000 s-1 to 0.01 s-1 at 30 °C. 30 test points were obtained. (4) Emulsions at different CO2 injection times were selected, and then CaO was added into the emulsions. The changes of the emulsions with CaO added in conductivity, emulsion breaking voltage, pH value and rheological properties were measured using conductivity meter, emulsion breaking voltage meter, precision pH test paper and HAAKE MARSⅢ rotary rheometer, respectively. The water solubility of the emulsions was observed too.
Fig. 2.
Fig. 2.
Diagram of CO2 bubbling device.
1.2.1. Emulsion inversion
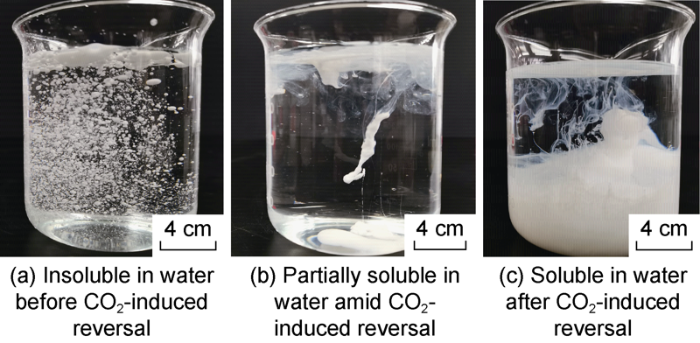
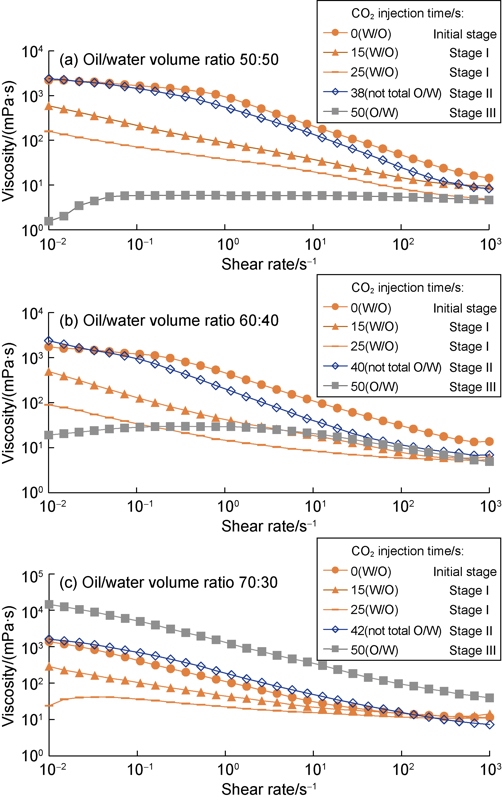
As shown in Figs. 3 and 4, with the CO2 injection time extending to 45 seconds, the amine emulsion increased from less than 0.01 μS/cm to more than 1 000 μS/cm in electrical conductivity, decreased from about 800 V to less than 10 V in emulsion breaking voltage, and evolved from insoluble, to partially soluble (transient), and completely soluble in water. These results show that the amine emulsion is able to reverse from water-in-oil state to oil-in-water state. When the volume ratio of oil to water changed from 50:50 to 60:40 and 70:30, the amine emulsion remained the inversion property, only lagged slightly in inversion.
Fig. 3.
Fig. 3.
Electrical characteristics and water solubility of amine emulsions with the CO2 injection time.
Fig. 4.
Fig. 4.
Water-soluble states of amine emulsion in different stages of CO2-induced inversion.
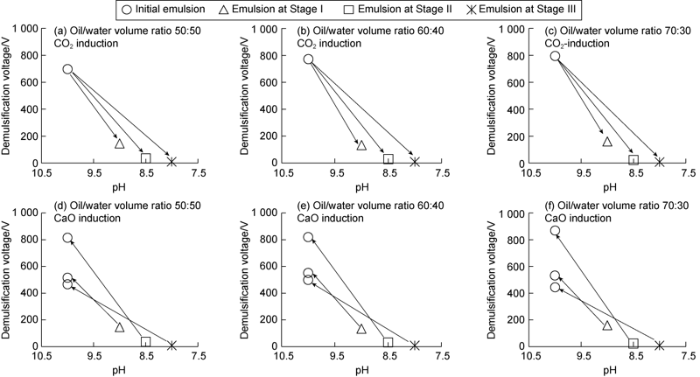
According to water solubility, emulsion breaking voltage and conductivity, CO2-induced emulsion inversion can be generally divided into three stages, insoluble in water (Stage I), partially soluble in water (Stage II) and completely soluble in water (Stage III), as shown in Fig. 3. During the inversion, the pH value of amine emulsion dropped from 10 to 8, and the pH values of the emulsion in Stage I, Stage II and Stage III were about 9.0, 8.5 and 8.0, respectively (Fig. 5a-c).
Fig. 5.
Fig. 5.
Changes in demulsification voltage and pH value under CO2/CaO effects.
Emulsions in Stage I, Stage II and Stage III were collected and were stimulated to turn back using CaO. As shown in Fig. 5d-f, the demulsification voltages went back to 515-550 V, 815-869 V and 445-500 V respectively as the pH value of the emulsions returned to 10 under induction of CaO. This indicates that the amine emulsions can reverse from water-in-oil state to oil-in-water state under the induction of CO2, and then can turn back to the stable water-in-oil state under the induction of CaO commonly used in oil-based drilling fluid.
1.2.2. Rheological transition
As shown in Fig. 6, the emulsion inversion was accompanied by the rheological transition. Herein, the emulsion with an oil/water volume ratio of 50:50 is taken as an example to illustrate. Shown in Fig. 6a, the rheological transition can be basically divided into three stages: (1) At stage I, the emulsion was still in water-insoluble water-in-oil state and dropped sharply in viscosity in the whole shear range of 0.01s-1 to 1000 s-1 with the increase of CO2 injection time. (2) At stage II, the emulsion was partially water-soluble and rose in viscosity in the shear rate range lower than 100 s-1, particularly in low shear rate range lower than 10 s-1. (3) At stage III, the emulsion was soluble in water completely and in oil-in-water state, compared with stage II, the emulsion dropped significantly in viscosity in the low shear rate range of less than 10 s-1. The emulsion with an oil/water ratio of 60:40 showed similar rheological transition (Fig. 6b). For the emulsion with an oil/water ratio of 70:30, the similar rheological transition behaviors were observed at Stage I and II, while the viscosity of the emulsion at Stage III increased higher than the initial level drastically during the whole shear rate range (Fig. 6c). The higher the oil-water volume ratio, the higher the viscosity at stage Ⅲ. The reason may be that the higher the proportion of oil phase (dispersed phase) was, the higher the viscosity of the emulsion system was.
Fig. 6.
Fig. 6.
Viscosity versus shear rate curves of amine emulsions at different CO2 injection times.
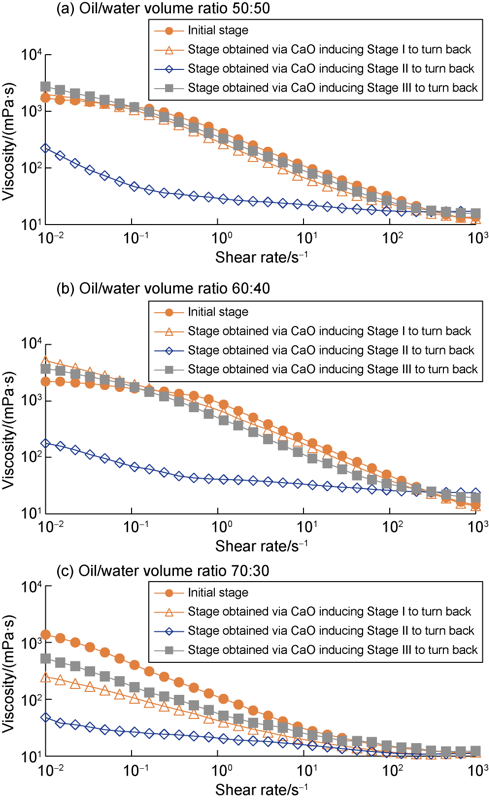
Amine emulsions reversed to Stage I, Stage II and Stage III were collected and treated with CaO until the emulsions turned back to 9.5-10.0 in pH value and above 450 V in emulsion-breaking voltage. Then, the rheological properties of the emulsions were measured on a HAKKE MARS III rotary meter. As shown in Fig. 7, the emulsions at Stage I and III had viscosities restored closed to the initial level during the whole shear rate range via CaO induction. As for the emulsion at stage II, the viscosity in the shear rate range above 100 s-1 restored closed to the initial level via CaO induction, while the viscosity in the shear rate range lower than 100 s-1 was obviously lower the initial level. This indicates that the amine emulsions which have reversed to oil-in-water state via CO2 induction can turn back to the initial water-in-oil state through CaO induction, meanwhile the emulsion viscosity can restore close to or lower than the initial level through CaO induction.
Fig. 7.
Fig. 7.
Viscosity versus shear rate plots of amine emulsions after CO2-inducing and CaO-inducing sequencely.
2. Formula and performance of oil-based drilling fluid
2.1. Formula
Amine emulsion was prepared by mixing 3# white oil, tertiary amine emulsifier and 25% CaCl2 brine. Stabilizing agent, barite and organic clay was added into the emulsion to get oil-based drilling fluids at oil/water volume ratios of 50:50, 60:40 or 70:30. The formula of the oil-based drilling fluids was 3# white oil + 5% emulsifier + 25%CaCl2 brine + 1.8% oil base stabilizer + 1% organic clay + 0.2%CaO+barite. The drilling fluids had a density of 1.4-2.0 g/cm3. The preparation procedure is: (1) The 3# white oil was mixed with the emulsifier and stirred at high speed for 1 min at room temperature, 25% CaCl2 was added slowly while stirring slowly, and then the mixture was stirred at high speed for 40 min at room temperature to get the CO2 responsive emulsion. (2) While stirring at high speed continuously, the oil-base stabilizer, barite, CaO and organic clay were added in turn into the emulsion, and the mixture was stirred for 40min to get the drilling fluid. The emulsifier used was the tertiary amine no-nionic surfactant shown in Fig. 1. The oil-base stabilizer was a reaction product of fatty acids, fatty amines and acid anhydride[29].
2.2. Basic performance
The settleability of solids in the drilling fluids was observed for 2 hours after the fluids were aged at 160 °C for 16 h. Then, the fluids were stirred at 10 000 r/min for 5 minutes, and the rheological properties of fluids were measured at 65 °C with a FANN® Model 35 A viscometer and the filtration properties were measured at 160 °C and 3.5 MPa with a high pressure/high temperature (HPHT) filtration device. The effects of oil/water ratio and fluid density on the fluid properties were analyzed.
As shown in Table 1, the drilling fluids with the oil/water ratios of 50:50, 60:40 and 70:30 and the densities of 1.4-2.0 g/cm3 after aging at 160 °C had emulsion-breaking voltages higher than 500 V, filtration loss less than 5 mL, and filter-cakes thinner than 3 mm, and no solid settlement. Their viscosity and filtration loss depended on the density and the water phase proportion more significantly. With the increase of density and water phase proportion, the viscosity and filtration loss inreased to some extent, but still were close to the application requirements.
Table 1 Basic properties of drilling fluids.
| Oil/water volume ratio | Density/ (g•cm-3) | Apparent viscosity/ (mPa•s) | Settling after ageing | Water loss/mL | Filter-cake thickness/mm | Emulsion- breaking voltage/V |
|---|---|---|---|---|---|---|
| 50∶50 | 1.4 | 50.0 | No | 1.6 | <1.0 | 636 |
| 1.6 | 60.5 | No | 2.0 | <1.0 | 679 | |
| 1.8 | 76.5 | No | 3.6 | 2.0 | 652 | |
| 2.0 | 94.5 | No | 4.8 | 3.0 | 528 | |
| 60∶40 | 1.4 | 49.0 | No | 0.2 | <1.0 | 735 |
| 1.6 | 55.0 | No | 1.8 | <1.0 | 711 | |
| 1.8 | 64.5 | No | 2.8 | 1.5 | 673 | |
| 2.0 | 73.0 | No | 4.0 | 2.0 | 599 | |
| 70∶30 | 1.4 | 30.0 | No | 0.1 | <1.0 | 759 |
| 1.6 | 37.0 | No | 0.2 | <1.0 | 754 | |
| 1.8 | 43.0 | No | 1.0 | 1.0 | 771 | |
| 2.0 | 55.0 | No | 2.0 | 1.5 | 656 |
2.3. Cleanup characteristics
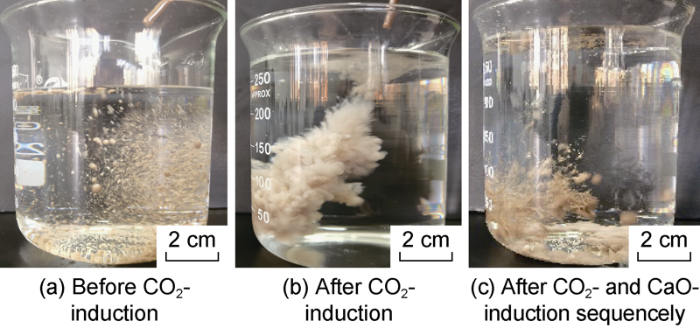
The CO2 responsiveness of the drilling fluids was investigated through water-solubility test, filter-cake and oily cutting cleanup tests. Herein, the drilling fluids with oil-water ratio of 60:40 and density of 1.4-2.0 g/cm3 are taken as an example to illustrate. 100mL of the drilling fluid was stirred at low speed continuously, and CO2 was injected into the fluid at 0.15 L/min for 5-6 minutes to induce emulsion inversion of the fluid (Fig. 8). It can be seen that the drilling fluid was insoluble in water before CO2 injection, after CO2 was injected, the drilling fluid dispersed rapidly in water and mixed with water, indicating that CO2 can induce the drilling fluid to change from water-in-oil state to oil-in-water state, making the drilling fluid easy to be washed.
Fig. 8.
Fig. 8.
Water-solubility test of the drilling fluid.
Then CaO was added into the drilling fluid treated by CO2, and the fluid returned to the water-insoluble state when its pH value recovered from 8.0-8.5 to 9.5-10.0 (Fig. 8c), indicating the drilling fluid reversed to the oil-in-water state through CO2-induction can turn back to the initial water-in-oil state through induction of CaO commonly used in oil-based drilling fluid.
The applications of CO2 response of drilling fluids in filter-cake cleanup and oily cuttings treatment were further investigated. The filter-cakes of the drilling fluids were built statically in a high-temperature/high-pressure equipment at 160 °C and 3.5 MPa, then they were soaked in 200mL fresh water respectively, and CO2 was injected into the water at the rate of 0.15 L/min. As the CO2 injection time increased, the filter-cakes gradually fell off the filter paper and dispersed in the water (Fig. 9).
Fig. 9.
Fig. 9.
Pictures of CO2-bubble cleaning of filter-cakes of drilling fluids with different densities.
Black shale cuttings collected from Silurian Longmaxi Formation of Weiyuan gas field were dried at 105 °C for 3 h (Fig. 10a), and then hot rolled in the different drilling fluids at 105 °C for 16 h respectively. The drilling fluids used included the oil-based fluid we prepared and the oil-based drilling fluid from Changning shale gas field.
Fig. 10.
Fig. 10.
Pictures of CO2 bubble cleaning of oily cuttings.
After rolling, the cuttings were soaked in the water respectively. We observed that the drilling fluids covering the cuttings’ surface could not be removed by water washing (Fig. 10b, e). Then CO2 was injected into the water, the comparison drilling fluid remained hydrophobic state and could not be removed from the cuttings’ surface (Fig. 10c-d), while the drilling fluid we prepared became hydrophilic and was readily removed by water washing (Fig. 10f-h).
2.4. Solid control
The solid control is for the purpose of removing useless solids with low density and size less than 0.075 mm[30]. Introduction of these solids in drilling fluid would cause drastic viscosity increase of the drilling fluid, reducing the penetration rate and hindering the reuse of drilling fluid. At present, it is hard to remove these useless solids from drilling fluid using the common vibrating screen method, and thinning agents are often needed to alleviate the impact of the useless solids on the properties of drilling fluid[31,32].
In this work, the useless solids of low density was removed efficiently by reducing the viscosity of the drilling fluid emulsion through CO2 induction. The specific experimental method is as follows: (1) Shale drilling cuttings were ground and passed through 200 mesh (0.075 mm) sieve to obtain the useless solids with low density and size less than 0.075 mm. (2) The cuttings powder with mass fraction of 25% was added into 300 g amine emulsion (with the oil and water volume ratio of 60:40 and 5% emulsifier). The cuttings/emulsion mixture was stirred evenly and rolled at 105 °C for 16 h. (3) CO2 was injected into the cuttings/emulsion mixture at 0.15 L/min in a bubbling way to induce viscosity drop of the emulsion. (4) The viscosities of the cuttings/emulsion mixture before and after CO2 injection were measured with a FANN® Model 35 A viscometer. (5) The settlement tendencies of useless solids in the mixture before and after CO2 injection were observed.
As shown in Table 2, for the initial emulsion without addition of useless solids, the pH value and emulsion breaking voltage gradually decreased with CO2 injection. As the pH value decreased to 9 and the emulsion breaking voltage decreased to about 300 V, the emulsion remained in water-in-oil state, but its viscosity dropped sharply. Especially, its yield stress dropped from 5.63 Pa to 0.51 Pa, and the disc reading Φ6/Φ3 on the viscometer dropped from 3.5/2.5 to 0.5/0, which was consistent with the results measured with the HAAKE MARSⅢ rotary rheometer. When 25 wt% of useless solids was added in the initial emulsion, the emulsion viscosity obviously increased; especially, the plastic viscosity of the mixture increased from 18.0 mPa∙s to 37 mPa∙s, indicating that the addition of useless solids caused obvious thickening of the emulsion system. Next, the initial emulsion contaminated with the useless solids was treated through CO2 bubbling until the pH value reduced to 9 and the emulsion breaking voltage reduced to about 300 V. The viscosity of the emulsion/cutting system decreased dramatically; the yield stress dropped from 7.15 Pa to 1.02 Pa, and the disc reading Φ6/Φ3 on the viscometer dropped from 7.0/6.0 to 0.5/0.5.
Table 2 Effect of CO2 on the viscosity of the amine emulsion/useless solids system.
| Cutting content/% | CO2 injection time/s | Apparent viscosity/ (mPa•s) | Plastic viscosity/ (mPa•s) | Yield stress/ Pa | Yield stress/ plastic viscosity/ (Pa•(mPa•s)-1) | Dial reading of viscometer (Ф6/Ф3) | Emulsion- breaking voltage/V | pH value |
|---|---|---|---|---|---|---|---|---|
| 0 | 0 | 23.5 | 18.0 | 5.63 | 0.32 | 3.5/2.5 | 762 | 10 |
| 15 | 20.5 | 18.5 | 2.04 | 0.11 | 1.5/1.0 | 434 | 9 | |
| 25 | 16.5 | 16.0 | 0.51 | 0.03 | 0.5/0 | 311 | 9 | |
| 25 | 0 | 44.0 | 37.0 | 7.15 | 0.19 | 7.0/6.0 | 753 | 10 |
| 15 | 33.0 | 30.0 | 3.06 | 0.10 | 2.0/1.5 | 399 | 9 | |
| 25 | 30.5 | 29.5 | 1.02 | 0.03 | 0.5/0.5 | 305 | 9 |
The settlement tendencies of useless solids in the emulsion before and after CO2 injection were observed. Prior to CO2 injection, little solids in emulsion system settled even after the system was set aside for 12 hours (Fig. 11a). After CO2 injection, approximate 3/4 of emulsion dropped significantly in cutting density after one hour of static settlement (Fig. 11b). Approximate 1/2 of emulsion became pure white after 5 hours of static settlement, indicating that the solids were completely removed via settlement (Fig. 11c). The white emulsion in the upper part of Fig. 11c was collected. Tests showed it had an emulsion breaking voltage of 290-310 V and pH value of 9. Next, CaO was added in this emulsion till its pH value returned to 10, then the emulsion breaking voltage of the system was tested, and the results were 550-580 V. This means that the useless solids can be removed via CO2-induced viscosity reduction, and the rest emulsion phase can be restored to the original stability level by adding CaO, thus facilitating the recycling of drilling fluid.
Fig. 11.
Fig. 11.
Settling of cuttings before and after CO2 injection.
3. Mechanism
Chemical structure characteristics of the initial amine emulsifier, emulsifier after CO2 induction, and emulsifier after CO2/CaO induction were tested by Fourier Transform Infrared Spectrometer (FTIR). The size changes of the amine emulsion droplets during emulsion inversion were measured by laser particle analyzer. The results provided evidences for explaining the mechanisms of CO2/CaO-induced emulsion inversion, rheological transition, filter-cake and oily cutting cleanup, and solid control.
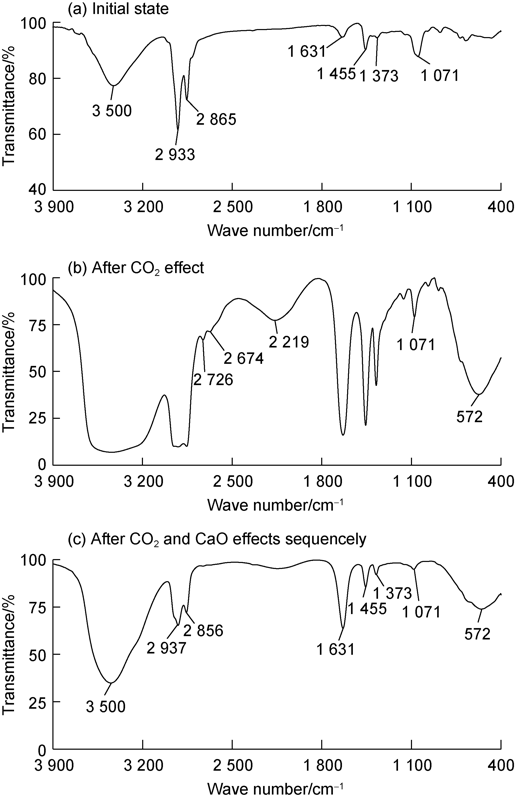
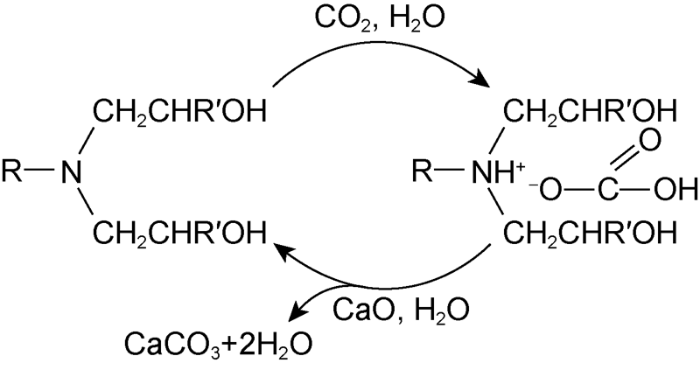
Fig. 12 shows the infrared spectra of emulsifiers in the three states. As shown in Fig. 12a, the infrared absorption peaks and bands of the initial emulsifier are characterized by: the single peak at 3 500 cm-1 attributed to the stretching vibrations of the O—H bond, two strong peaks near 2 933 cm-1 and 2 865 cm-1 attributed to the stretching vibrations of the C-H bonds, the peak at 1071 cm-1 attributed to the stretching vibrations of the C-N bond, and the peak between 1 900-1 200 cm-1 attributed to the bending vibrations of the C—H and O—H bonds. This indicates that the initial emulsifier belonged to organic amine. The emulsifier after reaction with CO2 still had the infrared absorption peaks and bands mentioned above, but had some obvious differences at 3 500 cm-1, 2 726 cm-1, 2 674 cm-1, 2 219 cm-1 and 572 cm-1. As shown in Fig. 12b, the peak at 3 500 cm-1 broadened, indicating there was hydroxyl groups’ association; broad peak and sharp peaks appeared near 2 200-2 750 cm-1, indicating the existence of tertiary ammonium salt; and an obvious absorption peak appeared near 572 cm-1, indicating the existence of O—C==O and C—C==O[33]. The analysis results of infrared peaks at 3 500 cm-1, 2 726 cm-1, 2 674 cm-1, 2219 cm-1 and 572 cm-1 show that the emulsifier changed from amine to amine salted under CO2 induction. After CaO was added, the broad peak and sharp peaks of the emulsifier near 2 200-2 750 cm-1 almost disappeared, indicating that the emulsifier turned from amine salt back to amine. In addition, the peak at 572 cm-1 in Fig. 12c remains obvious although much weaker than that in Fig. 12b. The reason is probably that there was CaCO3 remained in the test sample. Therefore, the chemical equation of CO2/CaO-induced reversible conversion between amine emulsifier and its salt is inferred as Fig. 13. As the amine salt is more hydrophilic than the amine, and the hydrophilic-lipophilic balance determines the type of emulsion formed, the emulsion stabilized by amine emulsifiers can change from water-in-oil state to oil-in-water state as the emulsifier becomes more hydrophilic under the induction of CO2. Furthermore, the oil-based drilling fluid formed based on the amine emulsion can change to water-based drilling fluid, and consequently is readily cleaned by water under the induction of CO2. When the amine salt turns back to amine via induction of CaO, the amine emulsifier regains the stronger lipophilic ability and the amine emulsion reverses from the oil-in-water state to water-in-oil state, facilitating the recycling of the drilling fluid.
Fig. 12.
Fig. 12.
Effects of CO2/CaO on the FTIR spectrum of the amine emulsifier.
Fig. 13.
Fig. 13.
CO2/CaO-induced reversible conversion between amine emulsifier and its salt.
As mentioned previously, the rheological behavior of amine emulsion shows regular transition accompanied with the emulsion inversion. As the emulsion reversed to Stage I under the induction of CO2 (Fig. 6), although the emulsion was still in water-insoluble water-in-oil state, its viscosity dropped sharply in the whole shear velocity of 0.01-1000 s-1. The sharp drop of viscosity can facilitate the removal of useless solids from the emulsion system.
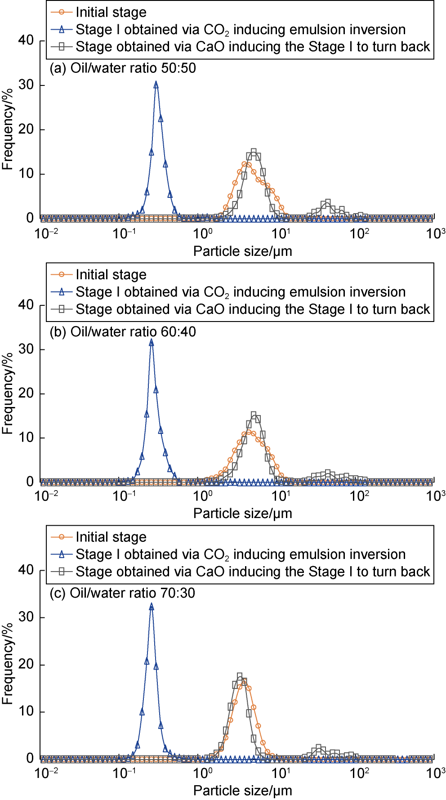
To investigate the mechanism of viscosity changes of the emulsion induced by CO2/CaO, a laser particle analyzer was used to measure the sizes of droplets of the emulsion at different stages (Fig. 14). The emulsion with the oil/water volume ratio of 50:50 is taken an example to illustrate here. The initial emulsion had a measured D50 of droplet size of approximate 4.7 μm. As the emulsion reversed to the stage I under CO2 induction, the emulsion remained in water-insoluble water-in-oil state, but the D50 of droplet size dropped to about 300 nm and the droplets became more homogeneous. When the emulsion of stage I was further treated by CaO, the D50 of droplet size increased from 300 nm to 5.1 μm. The emulsions with the oil/water volume ratios of 60:40 and 70:30 showed similar trends. Therefore, it can be concluded that CO2/CaO induces the rheological transition of the emulsion system mainly by regulating the sizes of the emulsion droplets.
Fig. 14.
Fig. 14.
Effects of CO2/CaO on the droplet size of amine emulsion.
In summary, CO2/CaO can induce the reversible conversion between amine emulsifiers and their salts, which appears as the inversion and rheological transition of amine emulsions macroscopically. If the amine emulsions are applied in the oil-based drilling fluid, the efficient cleanup of oil-based drilling fluid can be realized by CO2 induced emulsion inversion, the efficient removal of useless solids can be realized by viscosity drop of emulsion induced by CO2. Conversely, the rheological property and emulsion stability of the drilling fluid can return to the initial levels under the induction of CaO as the size of emulsion droplets and lipophilicity recover to the initial level. After this processing, the oil-based drilling fluid can be recycled.
4. Conclusions
Tertiary amine emulsions have CO2/CaO responsive property. They can have emulsion inversion and rheological transition via CO2 induction, while these CO2 responsive behaviors can be reversed by CaO.
The oil-based drilling fluids based on the tertiary amine emulsions not only remain the good drilling performance but also have reversible emulsion state and rheological behaviors controlled by CO2/CaO. They have unique edges in oily cutting treatment, filter-cake cleanup, useless solids removal and drilling fluid recycling, and are environment-friendly and low in cost. The oil-based drilling fluid based on the tertiary amine emulsion will have bright application prospects.
Nomenclature
D50—median particle size, μm;
R—C12-C22 linear carbon chain;
R'—H or CH3;
A'—O or NH;
m, n—number of related duplicate groups, 1-3;
Φ6—dial reading on a FANN® Model 35 A viscometer at the speed of 6 r/min, dimensionless;
Φ3—dial reading on a FANN® Model 35 A viscometer at the speed of 3 r/min, dimensionless.
Reference
Several views on accelerating the development of oil-based drilling fluid system in China
Geological characteristics and high production control factors of shale gas reservoirs in Silurian Longmaxi Formation, southern Sichuan Basin, SW China
Key technologies, engineering management and important suggestions of shale oil/gas development: Case study of a Duvernay shale project in Western Canada Sedimentary Basin
Achievements and future work of oil and gas production engineering of CNPC
Optimization and characterization of highly stable nanoemulsion for effective oil-based drilling fluid removal
DOI:10.2118/199904-PA URL [Cited within: 1]
Scale-up and design of a continuous microwave treatment system for the processing of oil-contaminated drill cuttings
DOI:10.1016/j.cherd.2009.07.011 URL [Cited within: 1]
New environmental friendly and harmless treatment technology for oil-based drilling cuttings of shale gas well
Evaluation of core damage caused by oil-based drilling and coring fluids
Wettability alteration caused by oil-based muds and mud components
DOI:10.2118/18162-PA URL [Cited within: 1]
Hole-cleaning capabilities of water-and oil- based drilling fluids: A comparative experimental study
DOI:10.2118/26328-PA URL [Cited within: 1]
Oil-based muds for reservoir drilling: Their performance and cleanup characteristics
DOI:10.2118/72063-PA URL [Cited within: 1]
Development of key additives for organoclay-free oil- based drilling mud and system performance evaluation
Offshore disposal of oil-based drilling-fluid waste: An environmentally acceptable solution
Super-amphiphobic, strong self-cleaning and high-efficiency water-based drilling fluids
Wettability alteration induced by oil based drilling fluid
Use of oil-based reservoir drilling fluids in open-hole horizontal gravel-packed completions: Damage mechanisms and how to avoid them
Reversible invert emulsion drilling fluids: A quantum leap in technology
DOI:10.2118/59479-PA URL [Cited within: 1]
New opportunities for the drilling industry through innovative emulsifier chemistry
Minimizing formation damage with a reversible invert emulsion drill-in fluid
Cleanup characteristics and mechanisms of reversible invert emulsion drilling fluid
DOI:10.1016/j.petrol.2015.06.021 URL [Cited within: 1]
Reversible invert emulsion system used to significantly increase water injection rates in an open hole, stand-alone screen completion in West Africa
Reversible drilling-fluid emulsions for improved well performance
Open-hole horizontal drilling and gravel-packing with oil-based fluids-an industry milestone
The reversible emulsion controlled by inorganic salt at high temperature or low permeability reservoir
Research status of environmentally stimuli-responsive emulsion systems
CO2 responsive emulsions: Generation and potential applications
Technologies and practice of CO2 flooding and sequestration in China
Technical difficulties and case study of oil base drilling fluid operation in shale gas drilling in south Sichuan
Disposing waste oil base drilling fluid from the Wei202H3 platform
IR fingerprint spectrum and its analyzing method