Introduction
The oil field development methods can basically be divided into three models in China, namely water flooding, chemical flooding and thermal recovery. With the deterioration of oil grade in China, the water flooding recovery factor is decreasing year by year. In recent years, gas injection development has been widely used gradually[1,2,3], such as carbon dioxide flooding, nitrogen flooding, natural gas flooding, flue gas flooding, and oxygen-reduced air flooding, etc., and pilot tests have been carried out in China. Air/oxygen-reduced air flooding has a significant gas source and cost advantage[4,5] compared with the flood-ing with natural gas, carbon dioxide, nitrogen and other gases, which are in short supply of gas source and with high cost. In addition, the low-temperature oxidation (LTO) reaction between air/oxygen-reduced air and crude oil is of great significance for enhancing the recovery rate.
Scholars have carried out extensive studies on the crude oil oxidation mechanism, oxygen consumption characteristics and enhanced oil recovery of air/oxygen-reduced air flooding. Kok et al.[6] studied the thermal kinetic characteristics of different crude oils by using the methods of Thermal Gravimetric (TG), Derivative Thermal Gravimetric (DTG) and Differential Scanning Calorimetry (DSC). They believed that light oil is more prone to LTO reaction. Ren et al.[7] carried out study through the combination of physical experiments with numerical simulation. They considered that the oxygen injected into the reservoir will be completely consumed, and a large amount of CO2 will be generated, thus forming a development mode similar to flue gas flooding, which can greatly improve oil recovery factor. Jiang et al.[8] believed that the enhanced oil recovery mechanism of LTO is mainly due to the formation of nitrogen drive after the oxygen is consumed by crude oil. Nitrogen can maintain the reservoir pressure, and the heat generated can reduce the viscosity of crude oil and make the volume of crude oil expand. The resulting CO2 dissolved in crude oil can also reduce the viscosity of crude oil. Hou et al.[9] carried out LTO experiments on light crude oil in low permeability reservoir, and obtained the kinetic parameters of LTO model, and improved the reaction kinetic model of LTO. Wang et al.[10] divided the oxidation of crude oil into four stages and made clear the oxidation characteristics of crude oil at different reaction stages. Liao et al.[4] discussed the oxidation characteristics of crude oil in the whole temperature domain, and suggested the reservoir types suitable for air/oxygen-reduced air flooding and the critical oxygen content of 10%[11]. However, at present, the study on the oxidation characteristics of crude oil in air/oxygen-reduced air flooding is mainly based on the oxidation thermal dynamics of crude oil and the static oxidation experiment of crude oil. There is little study on the oxidation characteristics of crude oil in porous media and the production law of remaining oil.
Light crude oil is used in this study: (1) Conduct TG-DSC combination analysis under different oxygen concentrations in order to make clear about the temperature limits of low-temperature oxidation of crude oil, and calculate the activation energy of different oxidation stages of crude oil; (2) With a tank static oxidation reaction device, conduct static oxidation experiments under a porous medium to make clear about the static oxidation characteristics and oxidation degree of crude oil; (3) Conduct Fourier transform ion cyclotron resonance (FT-ICR) and gas chromatography (GC-MS) analysis to study the mechanism of low-temperature oxidation in crude oil; (4) Carry out the dynamic displacement experiments of oxygen-reducing air and nitrogen, and analyze the production degree of crude oil in the pore throat with different scales by using the nuclear magnetic resonance method.
1. Experimental design
1.1. Samples and instruments
Light crude oil: From the the lower section of Paleogene Lower Gaskule Formation of Qinghai Oilfield (E31). After the sample is degassed, dehydrated, and the four components (SARA, i.e. saturated hydrocarbons, aromatic hydrocarbons, resin, asphaltene) were determined according to NB/SH/T0590—2010 standard[12]: the density of crude oil at 70 °C is 0.853 g/cm3, and saturated hydrocarbon, aromatic hydrocarbons, resin, asphaltene contents were 76.99%, 12.84%, 9.11% and 1.06%, respectively. C, H, O, N element contents are 74.70%, 12.59%, 9.31% and 3.40%, respectively. The Gaskule E31 reservoir is an abnormally high temperature, high pressure unsaturated reservoir, over the years of development, the current average pressure of the reservoir is 33 MPa, the average temperature is 126 °C, and the crude oil has small density, low viscosity, high degree of solidification point, high wax content[13]. Due to the high water saturation of the reservoir, it is currently planned to take the top oxygen-reduced air injection-assisted gravity flooding development[14].
Experimental gas: high pressure compressed nitrogen (purity of 99.99%), oxygen-reduced air with oxygen content of 5%, oxygen-reduced air with oxygen content of 10%, high pressure compressed air (oxygen content of 21%).
Static oxidized oil sand: each sample of oil sand used in static oxidation experiments is blended from 100 mL of dehydrated crude oil with 300 mL of quartz sand (80 to 100 mesh (0.150-0.180 mm)) (referred to as "8010" quartz sand).
Core: core samples from the Gaskule E31 reservoir were drilled, polished, washed and dried. Core samples have length of 30 cm, diameter of 3.8 cm, porosity of 13.6%, permeability of 93×10-3 μm2.
Experimental equipments: including thremo gravimeric- differential scanning calorimetry analyzer, tank type high temperature-high pressure crude oil static oxidation reaction device (300 mL), long core displacement experimental device, nuclear magnetic resonance meter, gas/liquid chromatograph, Fourier transform ion cyclotron resonance, Brookfield viscometer, etc.
1.2. Methods
1.2.1. Thermal analysis experiment
Mixing the oil and quartz particles in the thermal analysis experiments can solve the problem that the sample is not easy to combustion, and the quartz particles can provide passages for gas diffusion[15]. The experimental steps are as follows: (1) Calibrating the TG-DSC coupled analyzer; (2) Mixing 20 mg of oil sample with 100 mg of "8010" quartz sand into the crucible, and then put into the TG-DSC coupled analyzer; (3) Select oxygen-reduced air with oxygen content of 5% into the crucible and heating up, with injected gas flow rate of 50 mL/min, heating rate of 5 °C/min, heating range 25-600 °C; (4) Record the remaining mass fraction of the oil sample and the heat absorption and release process, plot the TG and DSC curves of the sample during temperature rise; (5) Repeat the steps (1) and (2), and set the heating rate of 10, 15 °C/min, repeat the steps (3) and (4); (6) Repeat the steps (1) and (2), replace the injection oxygen-reduced air gas to oxygen-reduced air with oxygen content of 10% and air (oxygen content of 21%), repeat steps (3)-(5), complete all experiments. In order to eliminate accidental errors, ensure the accuracy of experimental results, each group of experiments were repeated 3 times.
1.2.2. Static oxidation experiment
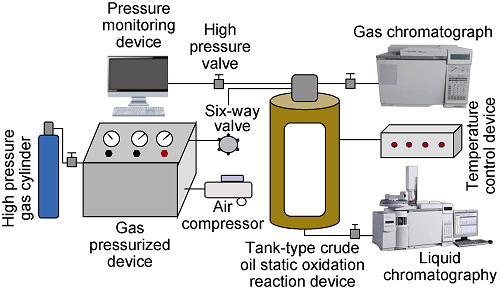
The experimental device (Fig. 1) is mainly composed of a high temperature and high pressure static oxidation reaction device, a gas pressurized device, a pressure monitoring device, and a gas/liquid phase analyzer. Among them, the high pressure reactor is manufactured by 316 steel, with a volume of 300 mL, temperature resistant to 350 °C, and pressure resistant to 70 MPa. The specific experimental steps are as follows: (1) Clean up the instrument to check the high pressure reactor gas tightness; (2) Add the static oxidized oil sand to the reactor and heated to the experimental temperature of 126°C. (3) Inject the oxygen-reduced air with oxygen content of 5% into the reactor, and turn off the injection valve when the pressure reaches the experimental pressure of 33 MPa. (4) Open the pressure monitoring device to record the pressure change in the reactor, and the experiment is over when the system pressure no longer reduced. (5) The gas samples in the reactor were collected and analyzed, and some oil sand samples were taken out for centrifugation, and the output oil sample was collected and made chromatographic analysis. (6) Repeat the steps (1) and (2), replace the injection gas to oxygen-reduced air with the oxygen content of 10%, air and nitrogen, repeat steps (3)-(5). (7) Replace the dehydrated crude oil to the reactor, repeat steps (1)-(6), and complete all the experiments.
Fig. 1.
Fig. 1.
Crude oil static low-temperature oxidation reaction device.
1.2.3. Dynamic displacement experiment
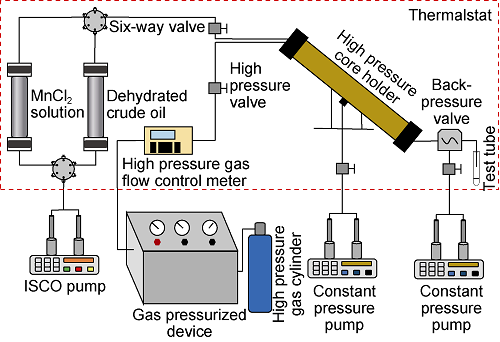
The experiment device is shown in Fig. 2. The core samples were used in oxygen-reduced air assisted gravity flooding experiments, and a group was carried out at the actual reservoir temperature of 126 °C, and the other group was carried out at 60 °C, with the experimental pressure of 33 MPa. The specific experimental steps are as follows: (1) The core is vacuumed and saturated with MnCl2 solution (mass fraction of 20%); (2) Using light crude oil samples to displace the oil phase to reach saturation (at this time, the water saturation in the core is a bound water saturation), and the nuclear magnetic resonance scanning is performed. (3) Choose nitrogen for displacement with the injection speed of 0.005 mL/min, end the experiment when 2 times pore volume is reached. Measure the amount of oil, calculate the recovery degree; (4) Scanning the core after displacement with nuclear magnetic resonance; (5) Clean and dry the core using ethanol, benzene solvent mixture with volume ratio of 1:3 core, repeat the step (1) and (2), replace the oxygen-reduced air with oxygen content of 10%, repeat steps (3) and (4) to complete all experiments.
Fig. 2.
Fig. 2.
Dynamic low-temperature oxidation displacement experimental device.
2. Oxidation kinetic characteristics of oil
2.1. Oil oxidation stage division
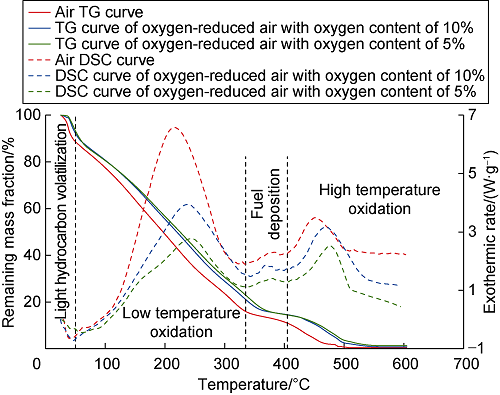
Due to the influence of thermal hysteresis, the temperature rise rate has effect on the oxidation stage division of the same oil sample. The higher the temperature rise rate, the higher the temperature division range of different oxidation phases[16]. The experimental results of the heat analysis experiment with temperature rise rate of 5 °C/min were selected as the basis for the division of the oxidation stage. With the rarefied experimental data, TG and DSC curves of the oil sample under different injection gas conditions were drawn (Fig. 3). It can be seen that when the injection gas is different, the peak of the DSC curve of the oil sample is different, but the temperature of the curve turning point and the overall trend are generally consistent.
Fig. 3.
Fig. 3.
TG and DSC curves of crude oil under different reaction gas conditions.
According to the peak and inflection point of the DSC curve, the whole process of crude oil oxidation can be divided into four stages: light hydrocarbon volatilization, low temperature oxidation (LTO), fuel deposition (FD) and high temperature oxidation (HTO), and the mass loss and exothermic data of the whole process of crude oil sample oxidation are shown in Table 1, and the mass change and exothermic law of crude oil in each stage have their own characteristics[17,18]. Take the reaction between crude oil and air as an example: (1) In light hydrocarbon volatilization stage, with the temperature below 60 °C and the mass loss of crude oil of nearly 10%, the oil sample is mainly lost due to distillation and volatilization, and the heat absorption effect is obvious (the exothermic rate is less than 0); (2) In LTO stage, this stage is dominated in the oxidation of crude oil in the full temperature domain, with the temperature of 60-335 °C and the mass loss of oil sample of about 70%. It is the main stage of mass loss. Due to the combined effect of oxygen addition and bond breaking reaction, the total exothermic heat increased. Therefore, the oil sample reacts with oxygen and releases heat to the environment. The exothermic effect of the oil sample at this stage is significant, and the first exothermic peak occurs when the temperature reaches 214 °C, with the exothermic rate reaching 6.58 W/g at this time; (3) In FD stage, with the temperature from 335 °C to 405 °C, the TG curve at this stage is smoother, and the exothermic situation is more stable, showing the second exothermic peak with smaller magnitude; (4) In HTO stage, the temperature was greater than 405 °C, and the third exothermic peak appeared when the temperature reached 449 °C, with an exothermic rate of 3.48 W/g. The exothermic peak at this stage was lower due to more mass loss of the sample in the early stage and less fuel remaining. Thermal cracking reactions generally occur in the FD and HTO stages, and the generated materials such as pyrolysis coke and light hydrocarbons[4] are helpful for subsequent ignition and combustion of oil reservoirs. The actual average temperature of Gaskule E31 reservoir is 126 °C, which is in the LTO temperature range. If the reservoir adopts the top oxygen-reduced air assisted gravity injection development, the crude oil oxidation kinetics features are mainly consistent with LTO.
Table 1 The mass loss and heat absorption and release parameters of crude oil reacting with gases with different oxygen contents.
| Reaction gas | LTO | FD | HTO | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tempera- ture range/ °C | Mass loss/ % | Peak tempera- ture/°C | Peak heat release rate/ (W•g-1) | Tempera- ture range/ °C | Mass loss/ % | Peak temperature/°C | Peak heat release rate/ (W•g-1) | Temperature range/ °C | Mass loss/ % | Peak temperature/°C | Peak heat release rate/ (W•g-1) | |
| Air (oxygen content of 21%) | 60-335 | 72.01 | 214 | 6.58 | 335-405 | 5.27 | 384 | 2.30 | 405-518 | 10.31 | 449 | 3.48 |
| Oxygen- reduced air with oxygen content of 10% | 62-345 | 69.08 | 236 | 3.94 | 345-410 | 4.70 | 370 | 1.87 | 410-560 | 11.47 | 468 | 3.19 |
| Oxygen- reduced air with oxygen content of 5% | 61-355 | 68.66 | 245 | 2.77 | 355-415 | 5.30 | 380 | 1.41 | 415-577 | 11.71 | 476 | 2.53 |
2.2. Characteristics of activation energy at different oxidation stages
The activation energy of crude oil when LTO occurs can reflect the ease of the reaction proceeding, and is very important for the study of chemical reaction rate and heat release per unit time. The temperature rise and oxidation of crude oil is a very complex physical and chemical reaction process, and the Flynn-Wall-Ozawa calculation method (referred to as the FWO method)[19] is chosen to avoid the selection of the reaction mechanism function and eliminate the error caused by the assumption of the reaction mechanism function, which is calculated by:
where
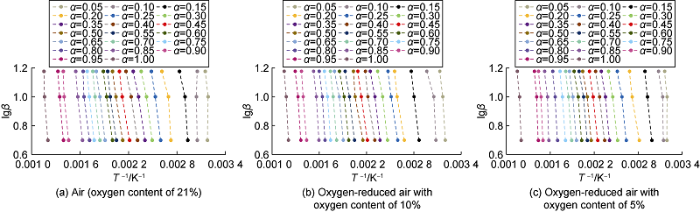
In Eq. (1), if the same value of α is chosen, $G\left( \alpha \right)$ remains unchanged, lgβ and 1/T will be linearly related, and activation energy can be calculated from the slope by linear fitting. According to the thermal analysis experimental data, the relationship curve between lgβ and 1/T was plotted using the FWO method (Fig. 4), and the average activation energy values of the three reaction stages under different experimental conditions were obtained by fitting (the fitted values of correlation coefficient squared were all greater than 0.9) (Table 2). It can be seen that, regardless of the gases involved in the reaction, activation energy required in the high temperature oxidation stage is the largest, followed by the fuel deposition stage, and the activation energy required in the low-temperature oxidation stage is the lowest. In different reaction stages, the oxygen concentration in the gases involved in the reaction is negatively correlated with the required average activation energy, and the higher the oxygen concentration, the lower the average activation energy required for the oxidation reaction. Compared with air (oxygen content of 21%), the activation energy required for the low-temperature oxidation of oxygen-reduced air with oxygen content of 10% is 5.46% higher, and the activation energy required for low-temperature oxidation of crude oil by oxygen-reduced air with oxygen content of 5% is 12.05% higher, which shows that the higher oxygen concentration in the reaction gas is more favorable for the oxidation reaction.
Fig. 4.
Fig. 4.
FWO relationship curves under different experimental conditions.
Table 2 Fitting results of average activation energy at different oxidation stages.
| Reaction stage | Average activation energy/(kJ•mol-1) | ||
|---|---|---|---|
| Air (oxygen content of 21%) | Oxygen-reduced air with oxygen content of 10% | Oxygen-reduced air with oxygen content of 5% | |
| LTO | 74.70 | 81.09 | 85.64 |
| FD | 102.74 | 126.86 | 138.50 |
| HTO | 135.50 | 139.21 | 151.59 |
3. LTO reaction characteristics
3.1. Comparison of static oxidation reaction characteristics of crude oil and oil sands
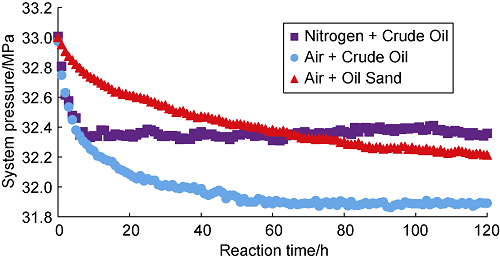
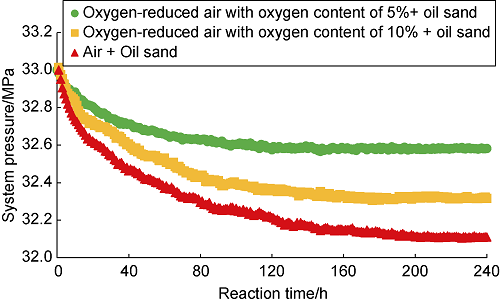
Li et al.[20] pointed out that under certain temperature conditions, when crude oil is in contact with temperature, violent dissolution and oxidation will occur between oil and air, and the pressure in the reactor will decrease rapidly and then stabilize. In fact, this phenomenon can also occur when nitrogen is injected into the reactor (Fig. 5). When air is injected into the reactor where the oil sand is placed, the pressure drop due to dissolution will be greatly reduced. The results of the static oxidation experiment of crude oil show that the pressure tends to be stable after 70 h of direct contact between air and crude oil. At this time, the oxygen is considered to be completely consumed. However, the reaction between air and oil sand is not the same. It will take longer time for the dissolution and oxidation of crude oil. After 120 h, the pressure still has a downward trend, indicating that the occurrence of LTO of the crude oil in the oil sand is not violent, but a process with the oxygen slowly consumed. Fig. 6 shows the pressure drop curve of the oxidation reaction between air and oil sands with different oxygen content. The higher the oxygen concentration in air, the higher the partial pressure of oxygen[21]. In the same crude oil contact area, the larger the volume fraction of oxygen in contact with the oil sample, the more oxygen dissolved in the crude oil, the more crude oil involved in the reaction. Therefore, the faster the oxidation reaction, the faster the pressure drops in light crude oil system. In Fig. 6, the pressure drop curve for the reaction between air and oil sand decreases the fastest, and the residual pressure of the system is the lowest, and the pressure drop curve for the reaction between oxygen-reduced air with oxygen content of 5% and oil sand decreases the slowest, and the residual pressure of the system is the highest.
Fig. 5.
Fig. 5.
Pressure drop curves for the reactions of nitrogen and air with crude oil and oil sand.
Fig. 6.
Fig. 6.
Pressure drop curves of the reactions between air and oil sand with different oxygen contents.
3.2. Change in oil and gas composition after LTO
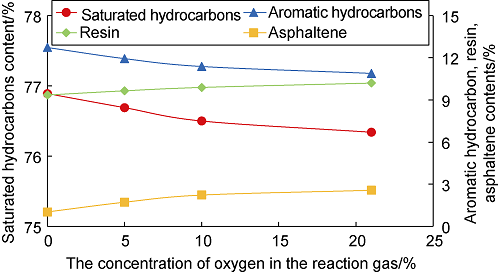
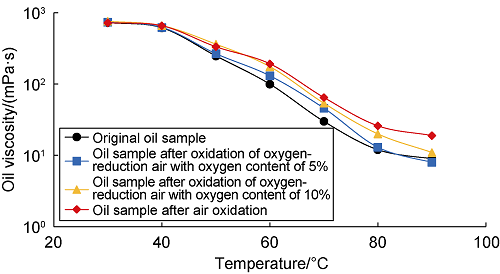
Fig. 7 shows the variation curves of the content of the four components in the oil sample with the oxygen concentration in the air after the oxidation reaction. As can be seen from the figure, the higher the oxygen concentration in the air, the stronger the LTO effect, and the lower the contents of saturated hydrocarbons and aromatic hydrocarbons in the oil sample after oxidation. When the oxygen concentration increased from 0 to 21%: (1) The content of aromatic hydrocarbons decreased from 12.73% to 10.89%, decreasing by 1.84%. The content of saturated hydrocarbons decreased from 76.89% to 76.34%, decreasing by 0.55%. It can be seen that the change of aromatic hydrocarbon content is greater, which is mainly because aromatic hydrocarbons are more reactive and easier to be oxidized than saturated hydrocarbons. (2) The resin and asphaltene contents both increased to different degrees, with the resin content increasing from 9.35% to 10.20%, an increase of 0.85%, and the asphaltene content increasing from 1.03% to 2.57%, an increase of 1.54%. (3) The crude oil viscosity increased accordingly due to the increase of resin and asphaltene contents (Fig. 8). It can be seen that the level of oxygen concentration not only determines the strength of LTO effect, but also brings a negative effect of the overall viscosity increase of crude oil.
Fig. 7.
Fig. 7.
The four-component change curve of crude oil reacted with air with different oxygen content.
Fig. 8.
Fig. 8.
Viscosity-temperature curve after reaction of crude oil with air of different oxygen content.
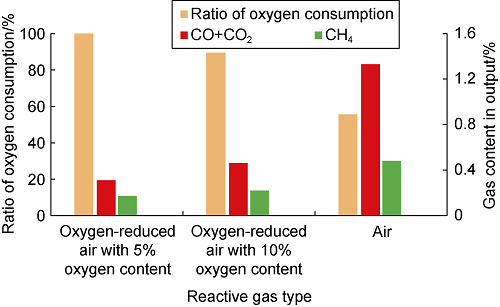
In actual field application, it is necessary to control the oxygen concentration during the injection to ensure that the oxygen concentration on the production end is lower than the explosion critical oxygen content. Therefore, it is of practical significance to study the oxygen consumption of LTO of crude oil. According to the static oxidation test results of crude oil, after the crude oil reacts with gases with different oxygen concentrations for 240 h, the oxygen consumption and the content of products are shown in Fig. 9. With the increase of oxygen concentration, the oxygen consumption ratio decreases, and the contents of CO+CO2 and CH4 in the output increase: (1) When oxygen-reduced air with oxygen content of 5% is injected, all oxygen is consumed, and 0.31% of (CO2+CO) and 0.17% of CH4 are generated. (2) When oxygen-reduced air with oxygen content of 10% is injected, oxygen consumption ratio is 89.4%, the residual oxygen concentration is 1.06%, and 0.46% of (CO2+CO) and 0.22% of CH4 are generated. (3) Inject air, the oxygen consumption ratio is 55.52%, and residual oxygen concentration is 9.34%. CO, CO2 and CH4 are generated from crude oil by LTO, and the mixed gas has an oil displacement effect similar to flue gas, which has a certain positive effect on enhancing oil recovery. The oxidation can also increase the viscosity of crude oil, and part of crude oil quality becomes worse. Therefore, it is more difficult for the crude oil to be recovered when the reservoir is subjected to air/oxygen-reduced air flooding, which has a certain negative effect on enhancing oil recovery, but the effect of enhancing oil recovery by flue gas drive is stronger.
Fig. 9.
Fig. 9.
Oxygen consumption and output after LTO with gas of different oxygen contents.
The above description is the result of static oxidation in the reactor. The experiment was carried out under constant temperature. On the one hand, the heat released by crude oil oxidation cannot be accumulated in the reactor. If under the reservoir condition, the reservoir temperature will rise due to the heat accumulation, thereby promoting the oxidation reaction, more oxygen will be consumed; on the other hand, inside the reactor, the gas phase is an excess phase relative to the oil phase, while the crude oil is an excess phase in the actual reservoir. In the actual reservoir, the oxygen consumption ratio of LTO in crude oil is higher than that in the experimental results[7, 22]. The experimental results can only reflect the change law between the oxygen concentration of injected gas, the oxygen consumption ratio and the content of products, thus the experimental data are not representative.
3.3. Variation of molecular composition of oxidized crude oil
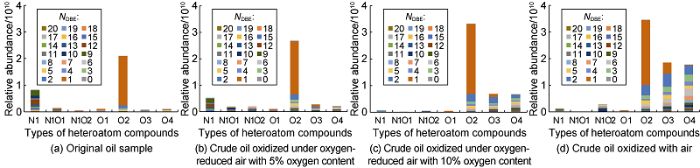
The molecular composition change of crude oil after LTO can be analyzed by FT-ICR technology. Fig. 10 shows the relative abundance distribution of alkaline heteroatom species (oxygen and nitrogen compounds) of the original oil sample and the oil sample oxidized by gas with different oxygen concentrations. Different colors in the Fig. 10 represent different NDBE (double bond equivalent number). The polar heteroatomic compounds in crude oil include N1, N1O1, N1O2, O1, O2, O3 and O4 compounds etc., among which the N1 and O2 compounds in the original oil samples are dominant in the component of crude oil. N1 compounds are carbazole-type non-alkaline nitrides, in which the content of N1 compounds with NDBE of 9-12 is higher, accounting for 64% of the total amount of N1 compounds with higher unsaturation degree; O2 compounds mainly consist of linear aliphatic, naphthenic and aromatic acids compounds[23]. In the original oil sample, compounds with NDBE of 1 or 2 in O2 compounds account for 90% of the total amount of these compounds, and are the main acidic substances in the oil sample. O2 compounds with NDBE of 1 are considered as chain saturated fatty acids. O2 compounds with NDBE of 2 are considered to be monocyclic naphthenic acids. The original oil sample also contains some O1 compounds (alcohols) with NDBE of 1, N1O1 compounds with low oxidation degree and N1O2/O3/O4 compounds with high oxidation degree, but with low content. N1O1 compounds are considered as intermediates of the oxidation of N1 compounds, and their functional group is hydroxyl[24].
Fig. 10.
Fig. 10.
Comparison of the abundance of heteroatom compounds in oil samples after oxidation of different reaction gases.
With the increase in oxygen concentration in the reaction gas, the degree of LTO increases gradually: (1) N1 and N1O1 compounds with lower oxidation degree tend to decrease, and the N1O2 compounds with higher oxidation degree increased relatively. When the reaction gas is air, the most N1O2 compounds are generated, indicating that more and more oxygen is fused to carbazole structure to promote the transformation of nitrogen-containing compounds[25]; (2) O1 compounds decreased gradually, and O2, O3 and O4 compounds occupied the dominant position gradually, indicating that alcohols are gradually oxidized and dehydrogenated to carboxylic acids[26]; (3) The chain saturated fatty acids with NDBE of 1 had bond broken and condensation effects to form naphthenic acids, and generated CH4, CO and CO2 as well. During the oxidation process, the content of chain acids decreased, and naphthenic acids became the main acidic components of crude oil after oxidation.
During LTO, a strong oxygen reaction occurs, and with the progress of the oxidation reaction, a large number of complex compounds containing multiple oxygen atoms are formed in the reaction products, most of which are phenols, aldehydes and ketones. O3 mainly represents carboxylic acid substances connected with a carbonyl group, and O4 represents complex oxidation products containing more oxygen, all of which are peroxide components. O3 and O4 compounds have strong polarity and are easy to adsorb on the surface of reservoir rocks, and may also change the wettability of rocks. It is difficult to recover residual oil with strong polarity in the subsequent gas injection development process. However, with the increase of temperature and oxidation reaction, some oxygen-containing derivatives will further undergo decarboxylation and decarbonylation reactions to generate more CO and CO2, and the reduction of polar substances is favorable for enhancing oil recovery subsequently.
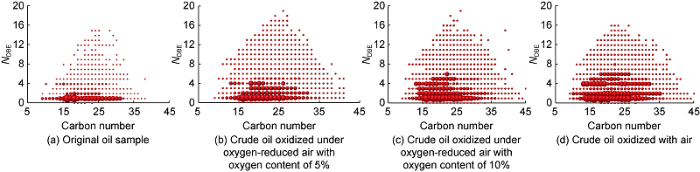
Fig. 11 shows the relationship between NDBE and carbon number of O2 compounds in crude oil after oxidation reaction between crude oil and air with different oxygen contents. It can be seen from the figure that the content of substances with NDBE of 1-4 is the highest among the four oil samples after oxidation, among which the content of C16 and C18 chain acids in the original oil sample is higher. As the degree of LTO increases, the NDBE of acids with different carbon numbers in the oil sample shows an increasing trend, and the content of naphthenic acid with NDBE of more than 1 gradually increases, which indicates that as the degree of LTO increases, more acid substances with complex structures are generated, and more chain acids are converted into cyclic acids.
Fig. 11.
Fig. 11.
The relationship between the double bond equivalent number and carbon number of O2 compounds after the oxidation reaction of crude oil and gas with different oxygen contents. The relative abundance of the compound in the figure is represented by the size of the sphere. The higher the relative abundance, the larger the volume of the sphere.
4. EOR mechanism of LTO reaction
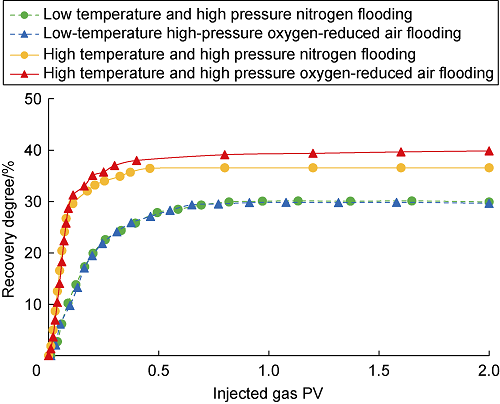
On the basis of the data from the dynamic displacement experiment, the relationship curve between pore volume multiple of injected gas and recovery degree was drawn (Fig. 12). At low temperature, LTO does not occur between oxygen-reduced air and crude oil, and the flooding effect of oxygen-reduced air is similar to that of nitrogen. Therefore, when the actual reservoir temperature is low, the oxygen in the injected air cannot be consumed effectively, and the oxygen content at the production end will be higher, which will lead to high risks of corrosion, explosion, etc. Under high temperature, the final recovery degree of oxygen-reduced air flooding is 39.86%, 3.30% higher than that of nitrogen drive (36.56%).
Fig. 12.
Fig. 12.
Dynamic displacement recovery degree curve.
Combined with the results of static oxidation test of crude oil, it is concluded that although the overall viscosity and heavy components of crude oil increase after LTO, the LTO effect can still improve effectively the oil recovery in dynamic displacement test, mainly because: (1) Although some light components of crude oil participate in the oxidation reaction in the static oxidation experiment, the gas is excessive phase, and the final viscosity of crude oil increases. However, in dynamic displacement, the crude oil is an excess phase, and the contact between the crude oil and air in the core is limited to the front of displacement, and the viscosity of crude oil in some parts increases and is located behind the displacement front (the displacement direction is forward), while the front of the edge of the displacement is not affected by this. On the contrary, the heat energy generated by the oxidation reaction is transferred to the front of the edge of displacement. On the one hand, the crude oil is heated and expanded, driving itself to flow to the production end; on the other hand, the viscosity of crude oil decreases when heated, which is favorable for the flowing of crude oil. (2) Through low-temperature oxidation, CO, CO2 and CH4 gases can be generated, forming flue gas drive in the reservoir, which has the functions of miscible phase, viscosity reduction and interfacial tension reduction to a certain extent[27,28]; (3) In the experiment, the core holding and the horizontal plane has degrees of incline, the production end is located below, and the driving effect of gravity also contributed to the improvement of recovery.
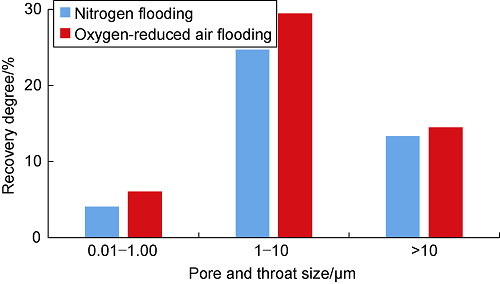
Air/oxygen-reduced air flooding has the functions of expansion, miscibility, viscosity reduction and interfacial tension reduction, so the advantages of air/oxygen-reduced air flooding are obvious compared with nitrogen drive. Fig. 13 shows the histogram comparing the pore throat recovery degree of different size intervals under the nitrogen and reduced oxygen air drive, which are obtained by nuclear magnetic resonance technology. For all sizes of pore throat intervals, the recovery degree of reduced oxygen air drive is higher than that of nitrogen drive. Therefore, under the condition of appropriate reservoir temperature, air/oxygen-reduced air flooding development should be preferentially adopted.
Fig. 13.
Fig. 13.
Recovery degree of pore-throat with different sizes by nitrogen and oxygen-reduced air flooding.
5. Conclusions
The whole oxidation process of crude oil can be divided into four stages, namely light hydrocarbon volatilization, low-temperature oxidation, fuel deposition and high-temperature oxidation, of which the highest activation energy is required at the high temperature oxidation stage, followed by the fuel deposition stage, and the lowest activation energy is required at the low-temperature oxidation stage. The oxygen concentration of the reaction gas is negatively correlated with the activation energy required for the reaction. The higher the oxygen concentration is, the lower the average activation energy required for the oxidation reaction.
The low-temperature oxidation reaction between crude oil and oxygen-containing air can produce a large amount of heat energy, at the same time, it also can produce CO, CO2, CH4, which forms flue gas drive in the reservoir, with the functions of miscible phase, viscosity reduction, interfacial tension reduction and oil expansion promotion. It is helpful to improve oil recovery.
In the case of suitable reservoir temperature, the recovery factor by oxygen-reduced air flooding is higher than that of nitrogen drive for pore-throat intervals with all sizes. Therefore, priority should be given to air/oxygen-reduced air flooding development.
Nomenclature
A—pre-exponential factor, s-1;
NDBE—number of double bonds equivalent;
E—activation energy required for oxidation, kJ/mol;
G(α)—kinetic model function in integral form;
f(α)—kinetic model function in differential form;
mf—final material raw quality, mg;
mi—initial raw material quality, mg;
mt—quality of raw material at a time, mg;
R—gas constant, 8.314 47 J/(mol•K);
T—absolute temperature, K;
α—percent conversion, %;
β—heating rate, K/min.
Reference
Status and enlightenment of international gas injection EOR technology
Experiments on nitrogen assisted gravity drainage in fractured-vuggy reservoirs
Technologies and practice of CO2 flooding and sequestration in China
Oil oxidation in the whole temperature regions during oil reservoir air injection and development methods
A guide to high pressure air injection (HPAI) based oil recovery
Thermal characteristics and kinetics of crude oils and SARA fractions
DOI:10.1016/j.tca.2013.07.014 URL [Cited within: 1]
Air injection LTO process: An IOR technique for light-oil reservoirs
DOI:10.2118/57005-PA URL [Cited within: 2]
Displacement mechanisms of air injection in low permeability reservoirs
DOI:10.1016/S1876-3804(10)60048-1 URL [Cited within: 1]
Kinetics of low temperature oxidation of light oil in air injection process
Oxidation reaction features of formation crude oil in air injection development
Applicable scope of oxygen-reduced air flooding and the limit of oxygen content
Test method for separation of asphalt into four fractions: NB/SH/T 0509—2010
Study on mechanism of low temperature oxidation reaction of deoxidized air drive: Taking E31 reservoir of Gaskule oilfield as an example
Pore-scale investigation of immiscible gas-assisted gravity drainage
DOI:10.1063/5.0033027 URL [Cited within: 1]
The impact of the oil character and quartz sands on the thermal behavior and kinetics of crude oil
DOI:10.1016/j.energy.2020.118573 URL [Cited within: 1]
Study of kinetic parameters of thermal decomposition of bagasse and sugarcane straw using Friedman and Ozawa-Flynn-Wall isoconversional methods
DOI:10.1007/s10973-013-3163-7 URL [Cited within: 1]
Comprehensive investigations into low temperature oxidation of heavy crude oil
DOI:10.1016/j.petrol.2018.08.027 URL [Cited within: 1]
Using DSC/TG/DTA techniques to re-evaluate the effect of clays on crude oil oxidation kinetics
DOI:10.1016/j.petrol.2015.07.014 URL [Cited within: 1]
Investigation on the low temperature oxidation of light oil for safely enhancing oil recovery at high temperatures and pressures
DOI:10.1016/j.energy.2020.117546 URL [Cited within: 1]
Experimental investigation on stable displacement mechanism and oil recovery enhancement of oxygen-reduced air assisted gravity drainage
New air injection technology for IOR operations in light and heavy oil reservoirs
Influence of biodegradation on crude oil acidity and carboxylic acid composition
DOI:10.1016/S0146-6380(00)00136-4 URL [Cited within: 1]
Microbial alteration of the acidic and neutral polar NSO compounds revealed by Fourier transform ion cyclotron resonance mass spectrometry
DOI:10.1016/j.orggeochem.2005.03.010 URL [Cited within: 1]
Exploring compositional changes along in situ combustion and their implications on emulsion stabilization via Fourier transform ion cyclotron resonance mass spectrometry (FT-ICR MS)
DOI:10.1021/acs.energyfuels.7b02421 URL [Cited within: 1]
Formation of carboxylic acids during aerobic biodegradation of crude oil and evidence of microbial oxidation of hopanes
DOI:10.1016/S0146-6380(02)00086-4 URL [Cited within: 1]
The contribution of low temperature oxidation of heavy crude oil to oil displacement efficiency during air injection in low permeability reservoirs
Low temperature oxidation of heavy oil in oxygen-reduced air: Effect of pressure and oxygen content on heat release
DOI:10.1016/j.petrol.2020.107957 URL [Cited within: 1]