Introduction
Gas hydrates are nonstoichiometric crystalline inclusion compounds with host framework built by water molecules and gas molecules entrapped in the cages of the framework[1]. Waxes are aliphatic, non-polar compounds with a high molecular mass. Both types of these compounds can pose a risk to flow assurance when crude oil is transported through pipelines, as they are capable of precipitating and depositing on the surface of the pipe walls. For gas hydrates to form, both gas and water must be present and the P-T conditions of the fluid should be within the stability boundary of hydrates (i.e. high pressures and low temperatures). Waxes precipitate from the oil phase when the temperature of the fluid decreases below a certain value - the Wax Appearance Temperature (WAT). Previous studies on gas hydrate and wax blocking the flow of crude oil were carried out separately, and there is no reference data yet on the interaction between these two compounds in pipelines. In 2008, Gao[2] studied the phenomenon of simultaneous precipitation of hydrate and wax from crude oil using a rocking cell and found that hydrate can promote the precipitation and deposition of wax. Mahabadian et al.[3] developed a thermodynamic model based on CPA (Cubic plus Association) equation of state[4], van der Waals-Platteeuw model[5] and UNIQUAC activity coefficient model to predict the phase behavior of wax and hydrate and experimentally proved that the formation of hydrate promotes the precipitation of wax under constant volume condition from thermodynamic point of view, because the light hydrocarbon in the oil phase is consumed during the formation of gas hydrate, which reduces the solubility of wax in the oil phase.
Wang et al.[6] studied the interaction mechanism of gas hydrate particles suspended in the waxy hydrocarbon phase. When the mass fraction of wax in hydrocarbon fluid is less than 5%, the wax deposition on the surface of hydrate particles enhances the hydrophobicity of hydrate surface and reduces the adhesion between particles. When the wax content exceeds 5%, the wax deposition will form sheeted shells around the hydrate particles that impede mass transfer between the gas molecules in the hydrate and the particle surfaces, slowing the rate of hydrate formation and creating more favorable conditions for the presence of a liquid layer on the surface of the hydrate particles. Thus, more water is capable to form capillary bridges between hydrate particles, which increases the cohesive force between them. Meanwhile, the interfacial tension between oil and water decreases with the increase of wax content[6]. Brown et al.[7] investigated the influence of wax on the adhesion forces between hydrate particles and stainless steel surface. It was found that the adhesion forces between hydrate and steel were significantly different for two waxes with different compositions. This result suggests that the adhesion forces may depend on nature of the deposited wax. Moreover, the tests on the performance of two different hydrate anti-agglomerants (AA) showed that in the presence of wax, the addition of one AA can decrease the hydrate adhesion to a steel surface, while the addition of the other AA can actually increase it. The results obtained in reference [7] show that if AA is chosen as a strategy to reduce the formation of hydrate plugs in pipelines, its performance should be checked taking into account the possible deposition of wax.
Some scientists have studied the influence of wax on the kinetics of hydrate formation in water-in-oil emulsions under shear conditions[8,9,10,11,12] and found that the induction time increases with increasing dissolved wax content in hydrocarbon media. This can be explained by the formation of a wax shell at the hydrate-water-oil interface. This wax shell can hinder the mass transfer of hydrate formers to water droplets or prevent the cross-nucleation of hydrate when hydrate particles collide with water. It has also been observed that the rate of conversion of water to hydrate increases with the increase of wax content under dynamic conditions[11]. Raman et al.[13] confirmed that wax can promote hydrate formation in the presence of surfactant under static conditions. Hovewer Chen et al.[11] found that the initial growth rate of the hydrate decreased with the increase of wax content in the oil under stirring conditions, which was attributed to the wax deposition at the oil-hydrate interface hindering the mass transfer of gas molecules to the hydrate. Presence of wax also affects the morphology and properties of hydrates in hydrocarbon media[11]. When wax was added to hydrocarbon media, viscous and soft jelly-like hydrates were formed, while hydrates formed without wax were hard and fragile.
Flowloop experiments have shown that the addition of a small amount of wax with a mass fraction of 0.75% dramatically increases the tendency of hydrate slurry in diesel oil to form plugs[14]. According to the analysis of pressure drop profile and flow rate profile, three scenarios of plug formation were outlined: gradual formation, transitional formation, and rapid formation. Low fluid temperature and low initial flow rate were found to be favorable conditions for rapid plug formation (sharp increase in pressure drop). Straume et al.[15] have shown that dispersion of hydrate particles can reduce the deposit thickness of wax on the pipe wall due to the abrasive effect of hydrate particles.
At present, most research focuses on preventing or mitigating of hydrate plugs formation [16,17,18,19,20] and developing models that can predict the risk of potential blockage in pipelines[17, 19-23], but studies on measures taken after plug formation are lacking. Sloan et al.[1] have summarized the conventional suggestions for treating gas hydrate plugs in pipelines, but more effective measures may be required to restore flow after the formation of mixed hydrate and wax plugs. de Oliveira et al.[24] extracted wax + hydrate plug from a rheometer after 24 hours of shut-in period, and found that the wax network can make the hydrate plug more stable under atmospheric conditions. However, this effect was observed only for one oil and not for the other oil. The processes of wax precipitation and hydrate formation are interrelated, and predecessors have proposed qualitative and quantitative models to describe these processes[3, 8, 11-12]. Considering that the study of the resulting agglomerates of wax precipitates and hydrates is helpful to optimize the schemes for the removal of the hydrate-wax plug, this work investigated the characteristics of the hydrate + wax plug obtained from an aqueous emulsion of crude oil with high wax content under quasi-static conditions. The decomposition of the hydrates contained in the plug was characterized and the rheological properties of the residual mixture after the decomposition of the hydrate were also studied.
1. Experiment
1.1. Materials
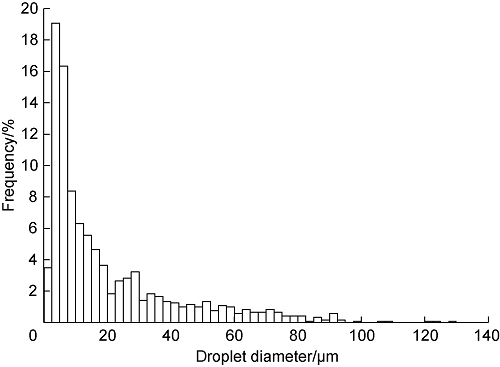
The experimental raw materials included crude oil from the East Siberian Oilfield, distilled water, and carbon dioxide with a purity of 99.99%. The mass fractions of each component of the crude oil are as follows: 86.3% saturated hydrocarbons (including 6.0% wax), 10.2% resins and 3.5% asphaltenes. The total acid number of the crude oil is 0.21 mg/g and the density is 841 kg/m3. Wax was added to the crude oil to increase the mass fraction of wax to 11.3% and to obtain the crude oil with high wax content. The mixture was heated to 333 K[25] and stirred continuously to provide dissolution of the wax. The mass fraction of wax was chosen by trial and error, and 11.3% was the lowest value that ensured plug formation in the laboratory. To prepare the emulsion with a water content of 50% the required amount of distilled water was added dropwise to the high wax crude oil and stirred at room temperature with a magnetic stirrer at a speed of 1000-1300 rpm for at least 3 hours. It is important to note that the crude oil could not form a stable water-in-oil emulsion without the addition of wax, and phase separation was rapid, but the addition of wax caused the emulsion to remain stable for a long time. The droplet size distribution of the emulsion was measured with an optical microscope by placing the sample on the glass surface. Over 1200 droplets were measured to build the droplet size distribution. Toupview[26] software was used to measure the droplet sizes. The diagram of droplet size distribution diagram drawn according to the measurement results is shown in Fig. 1.
Fig. 1
Fig. 1
Distribution of the size of water droplets in the emulsion.
1.2. Plug preparation device and process
The schematic of the apparatus for plug formation is shown in Fig. 2[27]. In the device, a liquid storage tank with an internal volume of 550 mL was connected to a tubular reactor, and the hydrate-wax plugs were formed in the tube. The tubular reactor had an inner diameter of 5 mm and a length of 250 mm, it was equipped with a cooling jacket, and the temperature in the tube was adjusted with a liquid thermostat. Pressure sensors were installed at the inlet and outlet of the pipe to record the pressure profiles at the inlet and outlet. The flow control valve downstream of the plug preparation device accurately controls the flow of fluid in the pipe by hand and prevents the plug from breaking due to a sudden change in pressure downstream of the plug. The fluid passed through the flow control valve was collected into a glass bottle. The experiments on plug formation were carried out as follows: First, the liquid storage tank was filled with emulsion. Second, the liquid storage tank was purged twice with 1 MPa of carbon dioxide to completely remove air from the system and pressurized to about 5.5 MPa (the total dilution factor in the device is more than 5000), and held for at least 12 hours to saturate the emulsion with carbon dioxide. Third, the temperature of the coolant was lowered and the shutoff and flow control valves were opened to push the saturated emulsion into the tube. Fourth, the flow rate of the emulsion in the tube was adjusted dropwise by the flow control valve. Typically the total volume of emulsion passed through the tubular reactor during the experiment was about 100-200 mL. If the downstream pressure stabilized at a level significantly lower than the upstream pressure and there was no breakthrough, the plug was considered to have formed. If breakthrough occurred, the flow control valve was closed, the setup was held for some time to reform the plug and the procedure was restarted. It should be noted that if the temperature was not lowered below 273.2 K in the third stage of the experiment we could not obtain any plug. Fifth, the temperature of the coolant was lowered to 253.2 K, the setup was depressurized, and the plug was knocked out of the tube as quickly as possible and placed in liquid nitrogen to prevent the hydrate from decomposing.
Fig. 2.
Fig. 2.
Schematic diagram of the experimental apparatus for plug formation.
1.3. Methods
The volume of gas released from the pieces of the plugs was determined with thermovolumetric gas gischarge experiments[28] and the characteristics of the decomposition process of the plugs were studied. A 0.3-0.5 g pieces of the plugs were placed in the chamber connected with the gas burette. All operations were carried out at the temperature of liquid nitrogen to avoid the decomposition of hydrates. Then, the chamber was heated at a rate of 1-2 K/min. The temperature of the chamber was measured with a K-type thermocouple. The emitted gas was collected in a graduated burette filled with saturated aqueous sodium chloride solution. Both the temperature of the chamber and the volume of the discharged gas were recorded. The hydrate content in the samples was calculated based on the volume of emitted gas and the mass of the decomposed sample, assuming the composition of the hydrate to be CO2·6.5H2O[29].
Scimitar FTS 2000 spectrometer was used to record Fourier transform infrared spectroscopy (FTIR). The measurement range was 400-4000 cm-1, and the resolution of the spectrometer was 4 cm-1. For the measurements, the samples were placed between KBr crystals. The spectra of the waxy crude oil and the plugs decomposition residues were measured respectively. The spectra were normalized to the values of maximum adsorption in each spectrum, which was at 1462 cm-1.
X-ray powder diffractograms were recorded using a Bruker D8 Advance diffractometer equipped with a TTK 450 Anton Paar cryogenic device at 2θ of 5°-45° in scanning mode of 2θ. The fully ground plug sample was placed in the pre-cooled to 173 K holder. Reflections of ice Ih (hexagonal ice), hydrate and solid wax were referenced relying on literature data[30,31,32,33].
The decomposition process of the plug was recorded by 50x optical microscope equipped with camera. The plug was placed in a homemade chamber with a cooling jacket connected to a thermostat to control the temperature of the sample. Transparent windows were located at the top and bottom of the chamber. Dry nitrogen was blown on the outer sides of the windows to prevent icing. At the beginning of the experiment the temperature of the chamber was 253.2 K. The chamber was heated to 293.2 K at a rate of 1-2 K/min and videos of the decomposition process were recorded.
The phase transitions of quenched plug samples in the temperature range 173.15-323.15 K were studied using the NETZSCH DSC 204 F1 Phoenix Differential Scanning Calorimeter. A small piece of the plug was placed in an aluminum crucible at the temperature of liquid nitrogen, then the crucible was sealed and placed in a calorimeter. The samples were heated at a constant rate of 9 K/min and the heat flow difference between the sample filled and empty crucible was recorded.
The rheological properties of plug decomposition residues (water and wax after hydrate decomposition) and waxy crude oil samples were tested using the HAAKE RheoStress 600 rheometer equipped with a PP20 titanium rotor. The samples and the rheometer were adjusted to the required temperature. Each sample went through two measuring stages: First, the shear rate was increased from 0.13 s-1 to 20 s-1 during 1 min in controlled strain mode, and the yield points and the flow curves of the fluid were obtained. Second, the viscosity of each sample was measured at a shear rate of 100 s-1 without changing the sample after recording the flow curve.
Deposition of components of the crude oil with high wax content (11.3%) was studied using a cold finger setup. 150 mL of the high wax crude oil was poured into the chamber. The chamber was purged 2 times with 1 MPa of carbon dioxide, pressurized to 5.5 MPa, and held for 24 h to ensure that the oil sample was saturated with gas at a temperature of 293.2 K. The temperature of the chamber was then adjusted to the necessary temperature. Then, the temperature of the container was raised to 333.2 K and held at this value for 4 h to ensure that all oil components were dissolved. After that, the temperature of the container was decreased to 293.2 K within 1 to 2 h and the finger temperature was set to 274.2 K. The experiment lasted for 8 h, during which the liquid phase was continuously stirred with a magnetic stirrer. After completion of the experiment, the deposits on the cold finger were scraped off and analyzed by FTIR spectroscopy to investigate whether the composition of the hydrocarbon phase in the plug formed by wax and hydrate was different from the composition of the cold finger deposits of crude oil obtained under similar experimental conditions.
2. Results and discussion
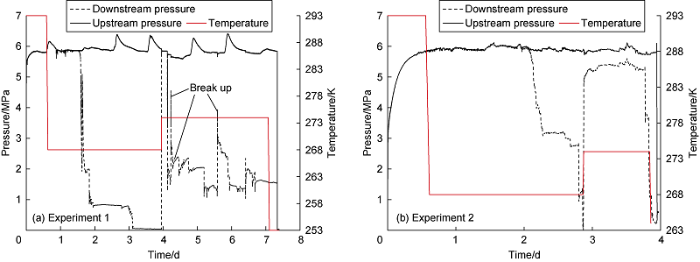
Three wax/gas hydrate plugs were collected at quasi-static experimental conditions described above. The pressure and temperature profiles for the performed experiments are shown in Fig. 3. In Experiment 1 (Fig. 3a), the pressure drop between the inlet and outlet of the apparatus increased for a long time at a temperature of 268 K and eventually stabilized. Increasing the temperature to 274 K resulted in several breakthroughs. Thereafter, the downstream pressure stabilized at about 1.5 MPa, clearly indicating a well-formed plug inside the apparatus. The plug was removed from the apparatus and turned out to be light colored and contained no dark oil inclusions, probably indicating about gradual deposition of the hydrate/wax mixture in the tube during this experiment. In the Experiment 2, the initial part was also performed at a temperature of 268 K (Fig. 3b). After the pressure at the outlet of the device decreased, the temperature of the apparatus was increased to 274 K. This operation resulted in a rapid increase in the pressure at the outlet to 5 MPa. This value of downstream pressure remained stable for 1 d and then dropped rapidly to almost zero, indicating the formation of a well-formed plug in this experiment. The rapid drop in the pressure difference between the inlet and outlet of the devices in Experiment 1 and Experiment 2 indicated that the plug formed in the first stage of the experiment contained ice. As the temperature increased above the melting point of ice, the ice began to melt and the pressure at the outlet increased rapidly as the plug lost its mechanical strength. This result shows that the mechanical stability of the formed plugs is provided by the solid phase of water and not by wax. In this case, the formation of a plug occurred in a short period of time due to the rapid formation of hydrate during ice melting. Most likely, numerous dark oil inclusions were found in the plug due to the comparatively rapid formation of the plug (Fig. 4b). The pressure-temperature curve and the shape of the plug obtained in the Experiment 3 were similar to those in the Experiment 2. Visual inspection revealed no hydrate inclusions noticeable in the resulting plugs. According to the data in reference[34], the size of hydrate particles formed in emulsions slightly exceeds the size of the droplets of the emulsion itself. We suggest that the size of hydrate particles in the obtained samples is also close to the size of the initial water droplets (Fig. 1) and is in the range of tens of μm. When some pieces of the plugs were heated to room temperature, the samples turned into a jelly-like mass with partially released water.
Fig. 3.
Fig. 3.
Pressure and temperature profiles of the plug formation experiments.
Fig. 4.
Fig. 4.
Photo of plug formation in Experiment 1(a) and Experiment 2(b). The scale below the sample is graduated in centimetres.
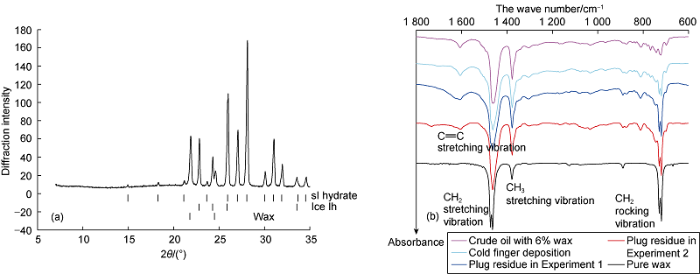
X-ray powder diffraction and FTIR spectroscopy were used to study the formed plugs. The typical diffraction pattern recorded at a temperature of 173 K is shown in Fig. 5a. The analysis of the diffraction patterns showed that all the samples contained three phases: Hydrate with cubic structure I (sI), which corresponds to the structure type expected for carbon dioxide hydrate, ice Ih and paraffin. Ice Ih was found in all samples, which could be due to the presence of some amount of unconverted water in the central part of the hydrate-covered water droplets or due to the partial decomposition of the hydrate during plug extraction. Thus, the X-ray diffraction data confirmed the existence of hydrate and solid wax in the plug samples.
Fig. 5.
Fig. 5.
X-ray diffraction pattern of a piece of the formed plug (vertical sticks correspond to the reflection position for the respective phases) (a) and FTIR spectra of different samples (b) in Experiment 1.
FTIR spectra of waxy crude oil, cold finger deposits from the waxy crude oil, residues of the plugs after decomposition of hydrate pure wax are shown in Fig. 5b. Five main adsorption bands are observed in the spectra: 1605 cm-1 corresponds to the stretching vibration of C==C, 1462 cm-1 and 1375 cm-1 correspond to the stretching vibration of CH2 and CH3 groups respectively, and 729 cm-1 and 720 cm-1 correspond to the rocking vibrations of the CH2 groups, which appear as doublets when the compound is in solid phase (solid wax)[35]. The C==O stretching vibration band at 1700 cm-1 is almost invisible, indicating that the polar compounds are present in the sample only to an insignificant extent.
Comparing the spectral coefficients (corresponding the ratio of band absorption intensity D), it can be observed that the obtained plugs are enriched with compounds containing poly -CH2- groups compared to the C==C groups and CH3 groups present in the initial crude oil, indicating the precipitation of wax from the oil in the hydrate-containing plug. At the same time, the plugs contain considerable amounts of other oil components; e.g. spectral coefficients C3 and P (Table 1) indicate the presence of aromatic compounds and branched hydrocarbons in the plugs residue and cold finger deposit. Quantitative analysis of these components is difficult, most likely due to the presence of residual water in the sample, which produces a background in the range 1300-1700 cm-1. Thus, according to the analysis of the FTIR spectra, the hydrate plugs we obtained contain significantly more solid wax than the initial wax solution in oil and the sediment obtained on the "cold finger".
Table 1 Spectral coefficients of waxy crude oil, pure wax, plug residues and cold finger deposits.
| Sample | C5 | C3 | P |
|---|---|---|---|
| Pure wax | 0.87 | 0 | 0.24 |
| Waxy crude oil | 0.28 | 0.16 | 0.64 |
| Experimental 1 plug | 0.80 | 0.24 | 0.67 |
| Experimental 2 plug | 0.79 | 0.09 | 0.48 |
| Cold finger deposits | 0.49 | 0.18 | 0.67 |
Note: C5=D720/D1462, represents the ratio of n-alkanes to total alkanes; C3=D1605/D1462 represents the ratio of aromatic hydrocarbons to total alkanes; P=D1375/D1462, represents the ratio of CH3 to CH2 in alkane structure, and indicates the degree of alkane branching.
The decomposition characteristics of the plug were studied by the thermovolumetry and Differential Scanning Calorimetry (DSC) methods. The hydrate content in the plugs was also estimated. The typical gas emission and DSC curves of the plug samples are shown in Fig. 6 (the curves shown were obtained with the plug from Experiment 1). In all cases the main part of gas (~60%) was emitted at temperatures near the ice melting point (273.2 K); a smaller portion of the gas was released at temperatures from 203 to 223 K. Due to the design of the plug formation setup, solid carbon dioxide cannot be present in the sample. Therefore, we attribute both stages of the gas emission to the decomposition of hydrates and the hydrate content in the plug was calculated based on the volume of carbon dioxide released (Table 2). Due to the heterogeneity of the plugs and the partial decomposition of some hydrates that might occur when the plugs were removed from the reactor, the hydrate contents in the different samples were different. A large endothermic peak with onset around 273 K can be seen on the DSC curves, corresponding to the decomposition of hydrates and melting of ice. In all cases, these peaks were followed by peaks corresponding to the melting of wax. Hence, the decomposition of hydrates and the melting of ice occur at temperatures at which the wax remains in the solid state. The absence of an endothermic peak in the temperature range 202-223 K is most likely related to the low heat of decomposition of the hydrate into ice and gas, as well as to the progress of the process in a wide temperature range. These experiments have confirmed that hydrate particles dispersed in wax can be effectively preserved below 270 K, and the results are consistent with earlier studies[36,37]. When the plugs formed by hydrate and wax are decomposed at low temperatures (as in northern regions), the self-preservation of hydrate particles dispersed in wax may play a crucial role.
Fig. 6.
Fig. 6.
The typical gas emission (a) and DSC (b) curves.
Table 2 Hydrate content in different plug samples.
| Experiment | Plug sample | Mass fraction of hydrate/% |
|---|---|---|
| Experiment 1 | Piece 1 | 0.28 |
| Piece 2 | 0.29 | |
| Experiment 2 | Piece 1 | 0.08 |
| Piece 2 | 0.14 | |
| Piece 3 | 0.32 | |
| Piece 4 | 0.41 | |
| Experiment 3 | Piece 1 | 0.31 |
| Piece 2 | 0.37 | |
| Piece 3 | 0.43 | |
| Piece 4 | 0.53 |
Note: The samples for gas emission experiments were taken from different pieces of obtained plugs randomly
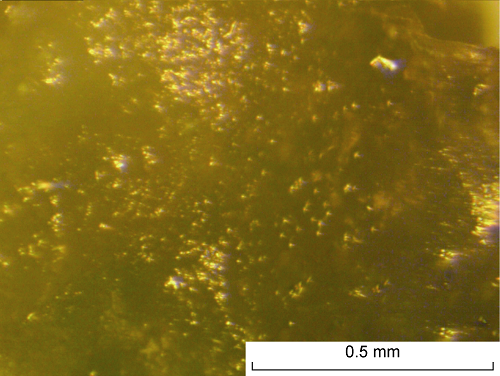
The decomposition process of the plugs was observed using an optical microscope in the temperature range 253-293 K with the pieces of plug obtained in Experiment 3. Before decomposition, the samples were translucent and turbid. Isolated black inclusions were visible, probably droplets of entrapped oil, and individual hydrate particles could not be distinguished. Decomposition was manifested by the appearance of numerous small gas bubbles in the volume of the sample (Fig. 7). The most intensive gas release was observed at temperatures from 270 K to 273 K. No significant changes were observed with the sample at temperatures 273-293 K and a gel-like substance was found at the place of the sample.
Fig. 7.
Fig. 7.
Optical micrograph photographs during the process of plug decomposition formed in Experiment 3.
The yield points and viscosity of the plugs residues at a constant shear rate of 100 s-1 were measured according to the procedures described above in the Experimental section at temperatures of 278 K and 293 K, and the results are shown in Table 3. It can be seen that the yield points of the plugs residues after hydrate decomposition were an order of magnitude higher than those of the waxy crude oil. The viscosities of the plugs residues were also much higher than the viscosities of oil with dissolved wax. This is undoubtedly due to a higher wax content in the plug residues. The rheological measurements of the plugs residues were carried out to understand the behavior of the fluid after the decomposition of the hydrate/wax plug at restart of the well.
Table 3 Yield points and viscosities of plug residues and waxy crude oil.
| Sample | Temperature/K | Yield points/Pa | Viscosity/(Pa•s) |
|---|---|---|---|
| Waxy crude oil | 293 | 262 | 0.169 |
| 278 | 2657 | 0.968 | |
| Plug residue | 293 | 441 (Experiment 2) | 1.392 (Experiment 1) |
| 278 | 6231 (Experiment 2) | 5.529 (Experiment 1) |
The results clearly show that even though the hydrates have decomposed, it can still be difficult to get the fluid to flow because a high yield stress is required to initiate the movement of the plug residue. The viscosity of the plug residues was an order of magnitude higher compared to viscosity of the oil with dissolved wax as well. Thus, the data of our work confirm the possibility of hydrate-wax plug formation in laboratory conditions. Since the oil used in this work is not in itself capable of forming stable emulsions with water, there is no doubt that the emulsion is stabilized by wax dissolved in oil. The small size of the hydrate particles in the suspension obtained from this emulsion indicates that the wax shell effectively prevents the coalescence of the droplets. At the same time, this shell allows the diffusion of gas into the droplet and thus the formation of a hydrate. In this case, the formation of a plug can be represented as the sticking together of hydrate particles coated with wax. It is known that the kinetics of hydrate formation and dissociation, hydrate density, and solubility of hydrate formers in oil and water differ significantly for carbon dioxide and methane. At the same time, many physical properties (including probably the properties of the surfaces) are quite similar for all hydrates and ice[38]. The same is true for the self-preservation of carbon dioxide and methane hydrates in oil dispersions[39]. In this work, we studied not the processes of formation of hydrate + paraffin plugs, but the properties of these plugs. Therefore, we can assume that carbon dioxide hydrate is a good model for methane hydrate in this case.
3. Conclusions
In this work, three wax/gas hydrate plugs were pre-pared at quasi-static conditions. We assume that these plugs might be laboratory models of hydrate/wax plugs formed in real pipelines at static or very slow flow conditions. Although all plugs were obtained at the first stage at 274.2 K, the temperature had to be lowered to 268.2 K; without this step we could not obtain the plug. It can be concluded that at quasi-static conditions, a temporary temperature decrease increases the risk of hydrate/wax plug formation. X-ray diffraction experiments, thermovolumetric gas emission experiments and FTIR experiments confirmed that the obtained plugs contained highly dispersed hydrate and solid wax. The components contained in the oil phase could effectively preserve the hydrate in the plugs, thus significantly reducing the decomposition rates of the hydrates in the plugs at subzero temperatures. After the decomposition of hydrates in the plugs, the yield stresses and viscosity of the plug residues were higher than those of waxy crude oil at the same conditions, which may have a certain influence on the restart of oil wells after plug formation.
Acknowledgments
The preparation of the plugs, the X-ray diffraction experiment, and the infrared spectroscopy experiment were supported by the Russian Science Foundation (17-17-01085). All other experiments were carried out within the framework of the Basic Research Programs of the Nikolaev Institute of Inorganic Chemistry SB RAS and the Institute of Petroleum Chemistry SB RAS.
Reference
Natural gas hydrates in flow assurance
Investigation of interactions between gas hydrates and several other flow assurance elements
DOI:10.1021/ef800189k URL [Cited within: 1]
Mutual effects of paraffin waxes and clathrate hydrates: A multiphase integrated thermodynamic model and experimental measurements
DOI:10.1016/j.fluid.2016.08.006 URL [Cited within: 2]
An equation of state for associating fluids
DOI:10.1021/ie9600203 URL [Cited within: 1]
Clathrate solutions
Influence of wax on cyclopentane clathrate hydrate cohesive forces and interfacial properties
DOI:10.1021/acs.energyfuels.9b03543 URL [Cited within: 2]
Effect of wax/anti-agglomerant interactions on hydrate depositing systems
DOI:10.1016/j.fuel.2019.116573 URL [Cited within: 2]
Effect of wax on hydrate formation in water-in-oil emulsions
DOI:10.1080/01932691.2019.1637751 URL [Cited within: 2]
An investigation on gas hydrate formation and slurry viscosity in the presence of wax crystals
DOI:10.1002/aic.v64.9 URL [Cited within: 1]
Induction time of hydrate formation in water-in-oil emulsions
DOI:10.1021/acs.iecr.7b01332 URL [Cited within: 1]
Experimental and theoretical investigation of the interaction between hydrate formation and wax precipitation in water-in-oil emulsions
DOI:10.1021/acs.energyfuels.8b01713 URL [Cited within: 5]
Study of hydrate formation in water-in-waxy oil emulsions considering heat transfer and mass transfer
DOI:10.1016/j.fuel.2019.02.014 URL [Cited within: 2]
Effect of particle hydrophobicity on hydrate formation in water-in-oil emulsions in the presence of wax
DOI:10.1021/acs.energyfuels.7b00092 URL [Cited within: 1]
Investigation of hydrate agglomeration and plugging mechanism in low-wax-content water-in-oil emulsion systems
DOI:10.1021/acs.energyfuels.8b01323 URL [Cited within: 1]
Perspectives on gas hydrates cold flow technology
DOI:10.1021/acs.energyfuels.8b02816 URL [Cited within: 1]
Wax and wax-hydrate deposition characteristics in single-, two-, and three-phase pipelines: A review
DOI:10.1021/acs.energyfuels.0c02749 URL [Cited within: 1]
Changing the hydrate management guidelines: From benchtop experiments to CSMHyK field simulations
DOI:10.1021/acs.energyfuels.0c01055 URL [Cited within: 2]
Interfacial phenomena in gas hydrate systems
DOI:10.1039/C5CS00791G URL [Cited within: 1]
Real-time estimation and management of hydrate plugging risk during deepwater gas well testing
DOI:10.2118/197151-PA URL [Cited within: 2]
A method for preventing hydrates from blocking flow during deep-water gas well testing
Hydrate deposition prediction model for deep-water gas wells under shut-in conditions
DOI:10.1016/j.fuel.2020.117944 URL
Prediction of hydrate deposition in pipelines to improve gas transportation efficiency and safety
DOI:10.1016/j.apenergy.2019.113521 URL
A new hydrate deposition prediction model for gas-dominated systems with free water
DOI:10.1016/j.ces.2017.01.030 URL [Cited within: 1]
Flow assurance study for waxy crude oils
DOI:10.1021/ef201407j URL [Cited within: 1]
Investigation of properties of the hydrate plugging and non-plugging oils
DOI:10.1080/01932690903224698 URL [Cited within: 1]
ToupView (Windows)/Dshow/Twain driver for microscope camera
(
Co-deposition of gas hydrate and oil wax from water-in- crude oil emulsion saturated with CO2
DOI:10.1088/1755-1315/193/1/012042 URL [Cited within: 1]
Gas hydrate of argon and methane synthesized at high pressure: Composition, thermal expansion and self-preservation
DOI:10.1021/jp053915e URL [Cited within: 1]
CO2 hydrate: Synthesis, composition, structure, dissociation behavior, and a comparison to structure I CH4 hydrate
DOI:10.1021/jp027391j URL [Cited within: 1]
Lattice constants and thermal expansion of H2O and D2O ice Ih between 10 and 265 K
Structure, composition, and thermal expansion of CO2 hydrate from single crystal X-ray diffraction measurements
DOI:10.1021/jp004389o URL [Cited within: 1]
X-Ray diffraction and electron microscope observation performed on various types of paraffins
DOI:10.1627/jpi1958.5.23 URL [Cited within: 1]
The normal paraffins revisited
DOI:10.1017/S0885715600015414 URL [Cited within: 1]
Direct conversion of water droplets to methane hydrate in crude oil
DOI:10.1016/j.ces.2009.08.013 URL [Cited within: 1]
IR spectra of basic classes of organic compounds
Unusual self-preservation of methane hydrate in oil suspensions
DOI:10.1021/ef401779d URL [Cited within: 1]
Influence of petroleum fractions on the process of methane hydrate self-preservation
DOI:10.1016/j.mencom.2018.09.028 URL [Cited within: 1]
Physical properties of gas hydrates: A review
Self-preservation of gas hydrate particles suspended in crude oils and liquid hydrocarbons: Role of preparation method, dispersion media, and hydrate former
DOI:10.1021/acs.energyfuels.6b01531 URL [Cited within: 1]