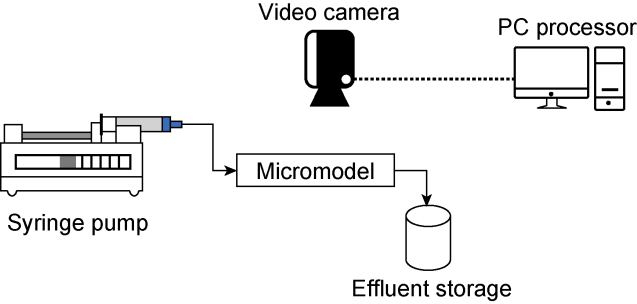
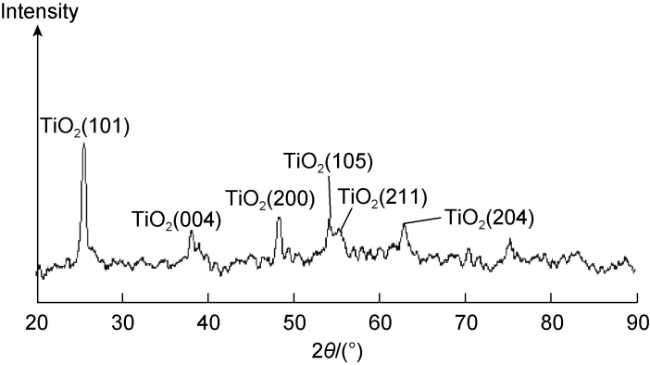
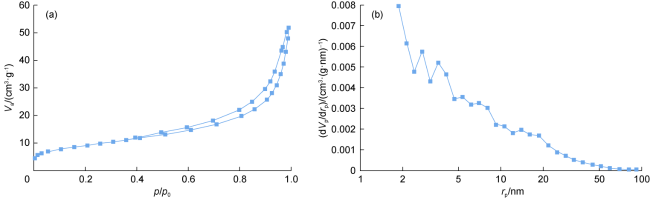
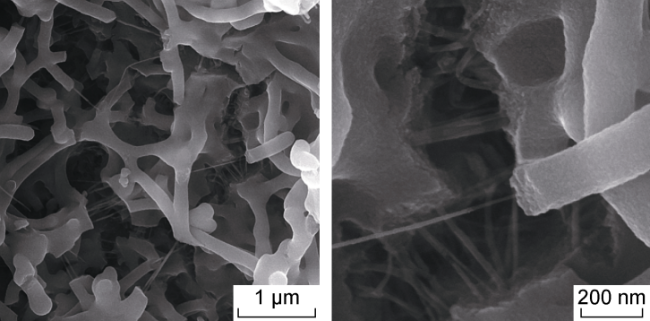
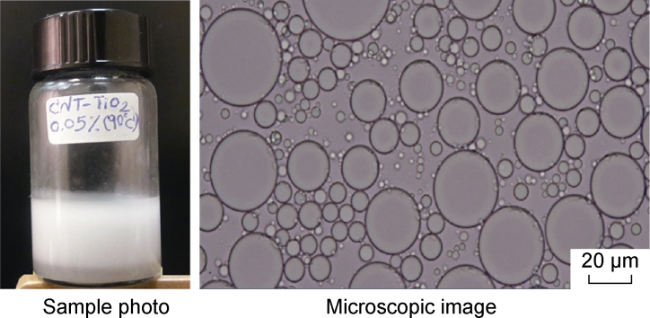
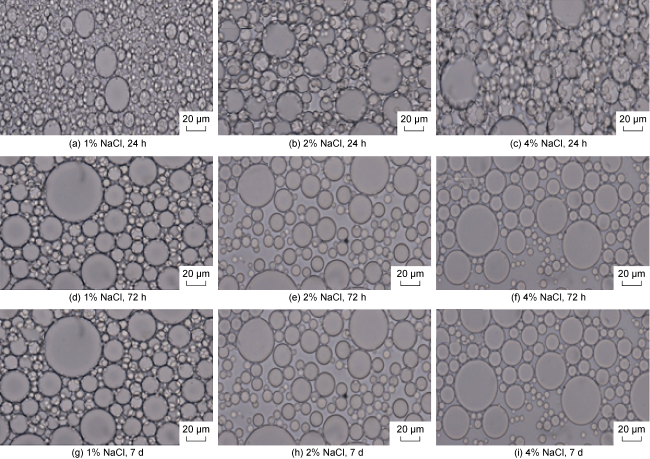
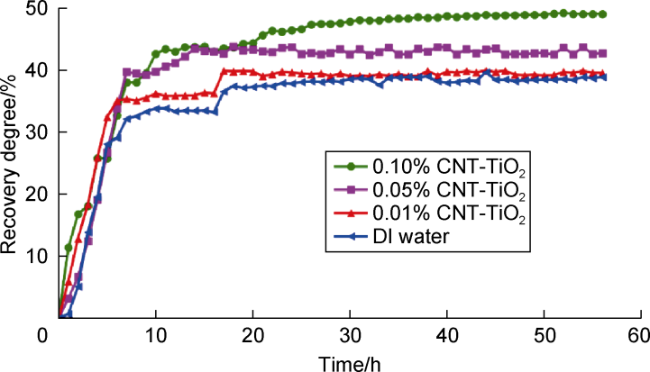
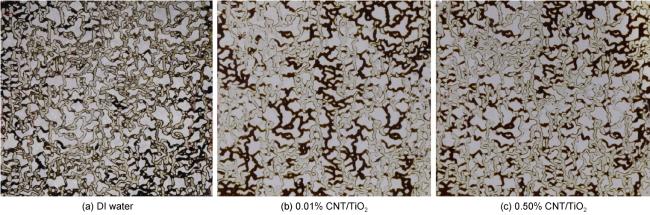
In recent years, with the advancement in nanotechnology and realization of its potential capabilities, researchers have conducted studies on the use of nanoparticles to enhance oil production
[4-5]. Pickering emulsion has been proposed as a new method of C-EOR where the stability of the emulsion is improved by employing solid particles
[6⇓⇓-9]. Silica and titanium are the most studied metallic nanoparticles for which it is confirmed that the synthesis conditions, functionalizing, and other factors can greatly impact their performance. Qin et al.
[10] proposed a novel method to in-situ synthesize silica nanoparticles in microemulsions with less tendency to agglomeration and a better Pickering formation activity and therefore the better performance in EOR. Yoon et al.
[11] stabilized the Pickering emulsion by using a colloidal layer that was composed of silica nanoparticles, dodecyl trimethyl ammonium bromide (DTAB) and poly (4-styrenesulfonic acid-co-maleic acid) sodium salt (PSS-co-MA). They observed that the colloidal dispersion raised the oil recovery factor by 4 percentage points in comparison to that of water flooding. In another work, Jia et al.
[12] stabilized the Pickering emulsions by dendritic silica nanoparticles and hybrids of dendritic mesoporous silica and titanium. In other attempts, Junus-SiO
2 nanoparticles
[13], AlO(OH) nanoparticles on sodium dodecyl benzene sulfonate
[14], mixed AlO(OH)/SiO
2 aqueous dispersions
[15], and silica nanoparticles/nonionic surfactant have been examined in stabilizing the Pickering emulsion and the EOR investigation. TiO
2 has also been a fascinating choice for EOR
[16-17]; however, TiO
2 has not been assessed in stabilizing the Pickering emulsions.