Ketzer et al.
[44] studied CO
2 water-rock interactions in saline formations in southern Brazil and confirmed that CO
2 could react with rocks to produce calcium carbonate for effective carbon sequestration under subsurface conditions. Mohamed et al.
[45] studied sulfate precipitation during CO
2 sequestration and suggested that temperature was the main parameter affecting sulfate precipitation and injection rate has no significant effect through comparative studies of temperature and injection rate. Even if the sulfuric acid concentration is low, calcium sulfate precipitation occurs under high salinity conditions; Liu et al.
[46] took into account the regional fluid flow while studying CO
2 sequestration in the Mt. Simon sandstone formation in the Midwestern U.S., and found a large amount of feldspar dissolution and clay mineral precipitation. Yu et al.
[47] studied the water-rock interaction in the displacement process of formation water saturated with CO
2 in the southern part of the Songliao Basin, and pointed out the variability of different mineral evolutionary characteristics: Calcite dissolved to the greatest extent, followed by smectite, iron dolomite was the weakest, and authigenic sodium feldspar and microcrystalline quartz did not undergo significant dissolution. Elkhoury et al.
[48] studied the dissolution and deformation of minerals in fractured carbonate reservoirs. Dávila et al.
[49] studied the problems related to CO
2 sequestration in high NaCl and sulfate-rich formation water in Hontomín of Spain, and systematically analyzed the change of Ca
2+, S
2−, Fe
2+ and Si
4+ before and after the reaction, pointing out that the dissolution of calcite, the precipitation of gypsum and the dissolution of small amounts of silicate are the main mineralogical changes. Taking the tight sandstone of the 7
th Member of Triassic Yanchang Formation in the Ordos Basin as an example, the author systematically analyzed the mineral and physical property changes during CO
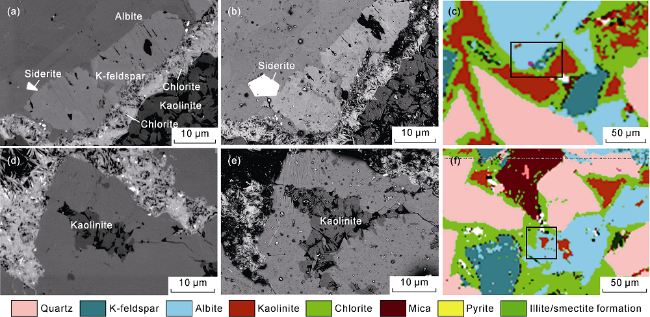
2 sequestration. It is indicated that that the dissolution intensity of potassium feldspar, sodium feldspar and calcite is the greatest, and the dissolution, migration and precipitation of clay minerals such as chlorite and kaolinite have important effects on the storage performance. As for the carbon fixation minerals, in addition to calcite, dolomite and montmorillonite, the author also found carbon fixation minerals such as rhodochrosite and kaolinite. From the in-situ comparison of field emission scanning electron microscopy before and after the CO
2 water-rock interaction, it can be seen that the particle size and morphology of rhodochrosite minerals shows a significant increase after the reaction (
Fig. 5a-5c), and the area of kaolinite distribution also shows a significant increase (
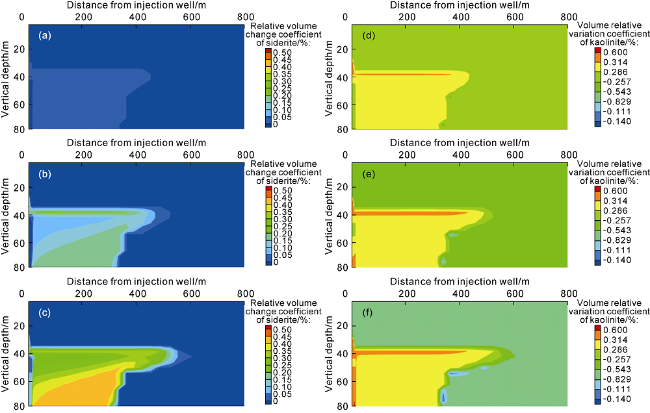
Fig. 5d-5f). Numerical simulation results of medium- and long-term supercritical CO
2 injection into sandstone strata further validate the contribution of kaolinite and rhodochrosite to carbon sequestration (
Fig. 6). With the increase of sequestration time from 200 to 1000 years, the distribution area of kaolinite and rhodochrosite in the precipitation zone gradually increases; at the sequestration time of 1000 years, the volume change coefficient (the difference between the instantaneous volume and the initial volume divided by the initial volume) of siderite in the precipitation zone is 0.004 50 (
Fig. 6c), the volume change coefficient of kaolinite is 0.002 86 (
Fig. 6f), and the distribution range can be as far as up to 600 m from the injection well.