Chelating agents can be used as chemical enhanced oil recovery fluids and reduce the salinity of injected water by capturing and chelating metal ions such as Ca
2+, Mg
2+ and Fe
2+ from fluid and rock and behave like LSW
[13,20⇓⇓ -23]. Chelating agents such as EDTA are stable at high temperatures up to 200 °C
[24]. They are not absorbed on the rock surface and do not damage the pores and also prevent precipitation by chelating different metal ions from rock and fluid
[25⇓⇓-28]. Fredd and Fogler
[29] were the first to use EDTA chelating agent as stimulation fluid in oil and gas fields, and Attia et al.
[13] also used EDTA chelating agent for the first time to enhance oil recovery. Chelating agents are compatible with sandstones and carbonate rocks and are available in different pH values. Previous studies have shown that using chelating agents at high pH increases oil recovery and improves the performance of chelating agents
[13,20,30⇓ -32]. Injecting chelating agents at high pH can disturb the initial equilibrium between crude oil and rock and increase oil recovery
[13,20]. Chelating agents can also be mixed with untreated SW, which is an effective way to reduce the costs of enhanced oil recovery and flooding projects
[13,30]. SW contains high concentrations of sulfate ions, while FW contains divalent ions such as Ba
2+, Ca
2+ and Sr
2+. By mixing FW with SW, an unstable solution is formed and precipitates CaSO
4, SrSO
4, BaSO
4 in the reservoir
[12,33]. Chelating agents capture metal ions and prevent them from reacting with other ions. Capturing metal ions from the solution prevents some problems, such as corrosion, precipitation and permeability reduction. Therefore, chelating agents in undiluted SW can behave the same as LSW and affect the rock dissolution and enhanced oil recovery process
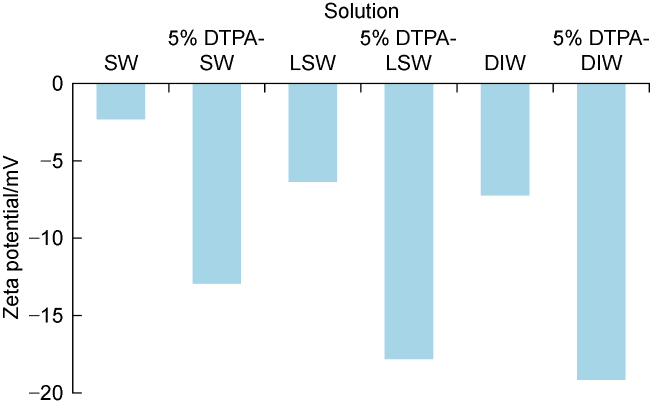
[34⇓-36]. However, unlike LSW, chelating agents prevent precipitation and do not cause any formation damage. For these reasons, chelating agents have been proposed as an alternative to LSW flooding
[13,35]. The dependence of the chelating agent on a metal ion is defined by the stability constant. High stability constant means high dependence of chelating agent on metal ions. For example, for all chelating agents, the stability constant with Fe
2+ is high, which means chelating agent first separates Fe
2+ from rock or solution and then separates other ions, such as Ca
2+ and Mg
2+ [21]. DTPA has the highest stability constant among all chelating agents, which means it takes ions from the solution more strongly and prevents ions precipitation
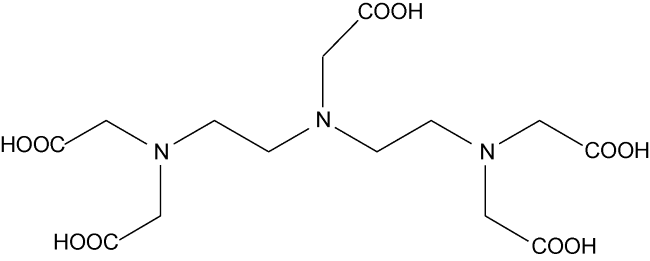
[32]. Also, compared to other chelating agents, DTPA has the most carboxylic groups in its structure
[37⇓⇓⇓-41]. The additional carboxylic group in DTPA solubilizes more oil and decreases the IFT. Because of these reasons, in this study, we used DTPA chelating agent to perform different experiments. Wettability is one of the essential parameters in reservoirs that controls the location, distribution and fluid flow in the porous media
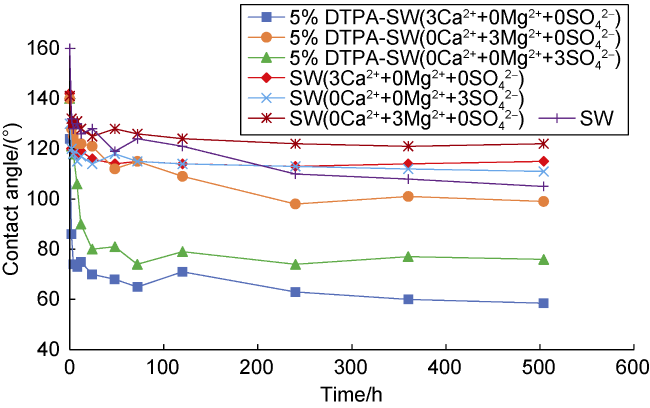
[42]. Wettability affects almost all parameters required for reservoir management, including relative permeability, electrical properties, saturation distribution and capillary pressure
[43]. Various methods are used to determine rock wettability alteration, including spontaneous imbibition tests, contact angle measurement, Zeta potential measurement, capillary pressure diagrams, relative permeability diagrams, and nuclear magnetic resonance. Ali
[44] reduced the rock/oil contact angle from 134° to 22° by mixing 1000 mg/L Fe
3O
4-mineral-soil nanocomposites within SW. Wettability alteration is the first oil recovery mechanism proposed using chelating agents as enhanced oil recovery fluids
[11]. Chelating agents at high pH separate various metal ions from solution, so the rock surface must release cations from its surface to achieve equilibrium. This dissolution process changes the rock wettability to more water-wet and with the release of ions from the rock surface, more oil will be recovered
[11,22⇓ -24,31,45 -46]. Mahmoud et al.
[47] found that rock dissolution, IFT reduction and wettability alteration are possible mechanisms to improve oil recovery using HEDTA chelating agent. They also stated that EDTA of mass fraction 5% (5% EDTA for short) flooding in limestone core and 5% HEDTA flooding in sandstone core recovered more than 20% of OOIP. Hassan et al.
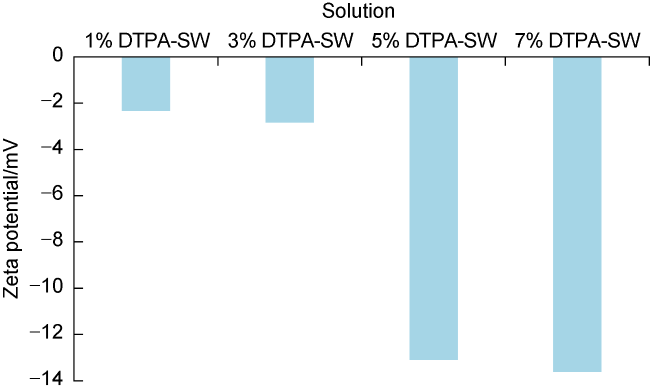
[48] performed sequential flooding of EDTA chelating agent in carbonate rocks. The CT scan and pressure drop results showed that high concentrations of chelating agent led to rock dissolution and wormhole creation. They recommended using EDTA chelating agent at a mass fraction of less than 10%. Zeta potential measurements also showed that increasing the concentration of chelating agent leads to more negative charges on limestone surface. Hassan et al.
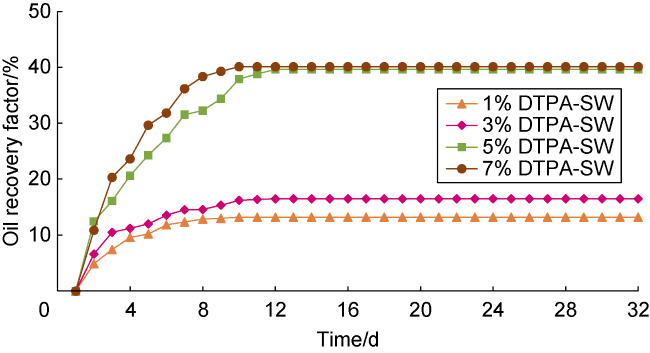
[49] investigated the performance of EDTA in enhanced oil recovery from carbonate samples. They considered 3% of EDTA chelating agent as the optimum concentration, which resulted in maximizing oil recovery without causing severe rock dissolution. Mahmoud et al.
[20,23] investigated the performance of EDTA in chelating Fe
2+ from iron-rich minerals and used EDTA chelating agent to enhance oil recovery from sandstone samples. They were able to increase oil recovery up to 19% of OOIP using 10% EDTA solution without any formation damage. Hassan and Al-Hashim
[50] showed that carbonate core flooding using EDTA solution altered wettability towards more water-wet. Also, ICP analysis confirmed that Ca
2+ were chelated from the rock during EDTA flooding and their concentration increased in flooding effluent.