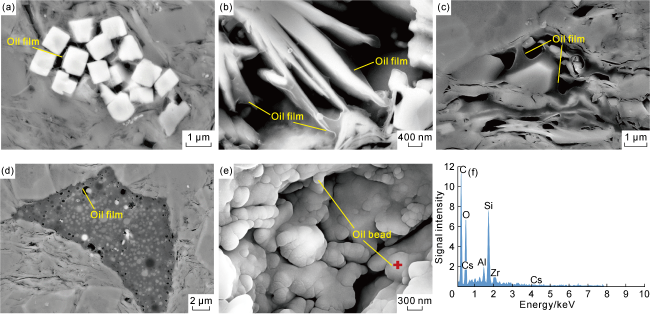
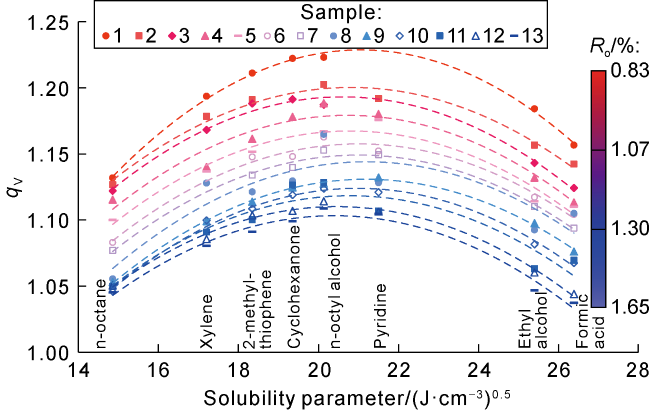
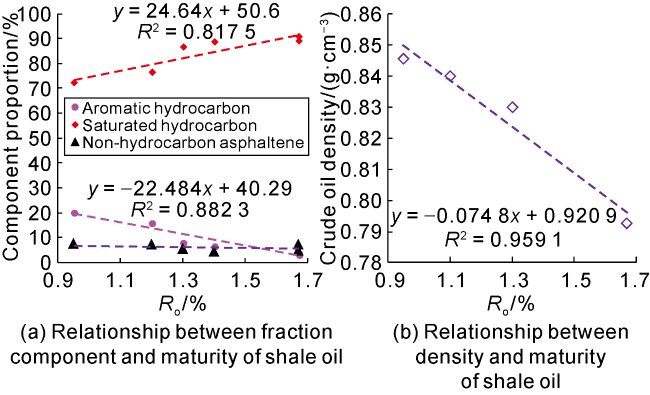
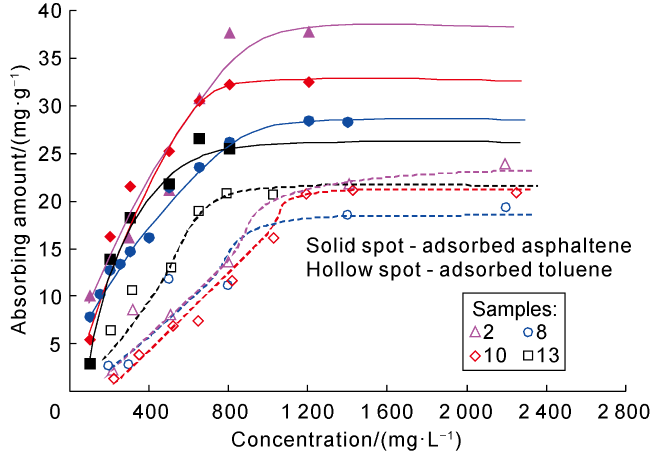
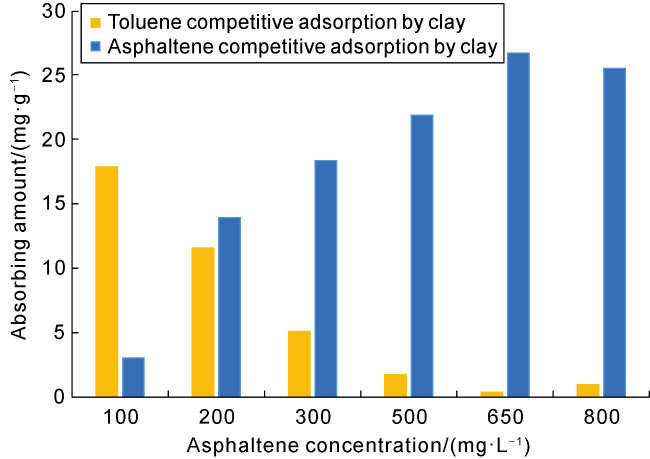
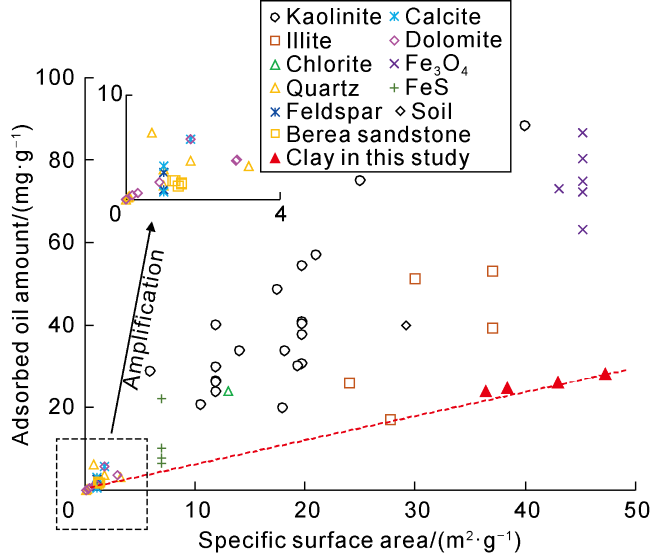
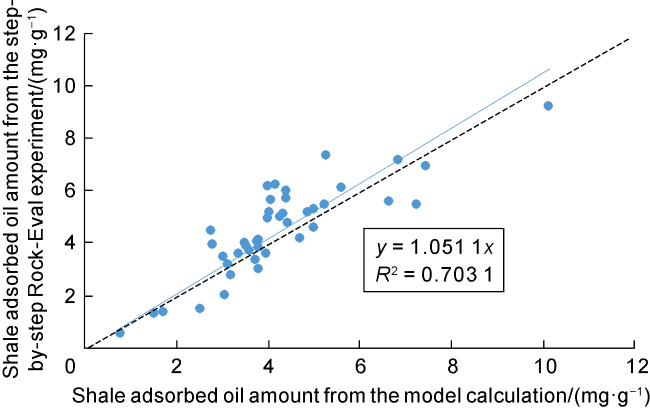
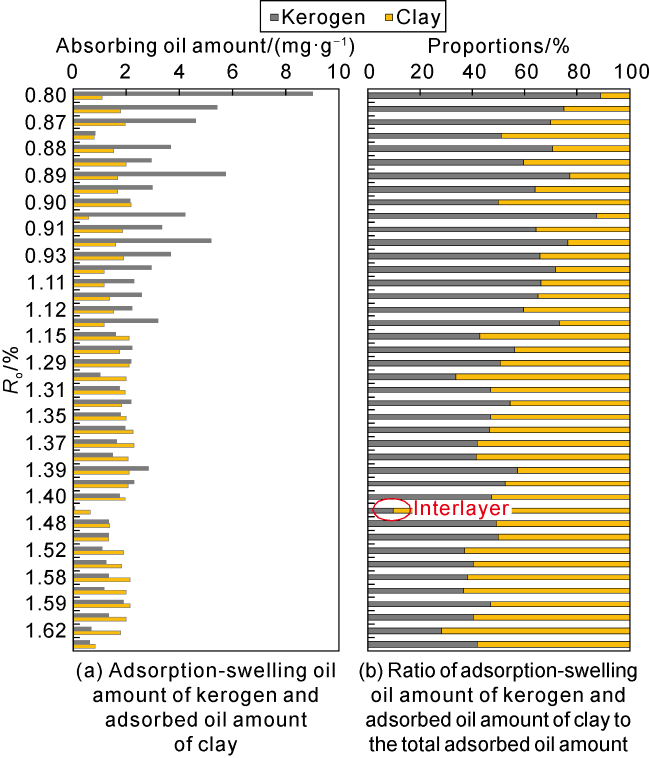
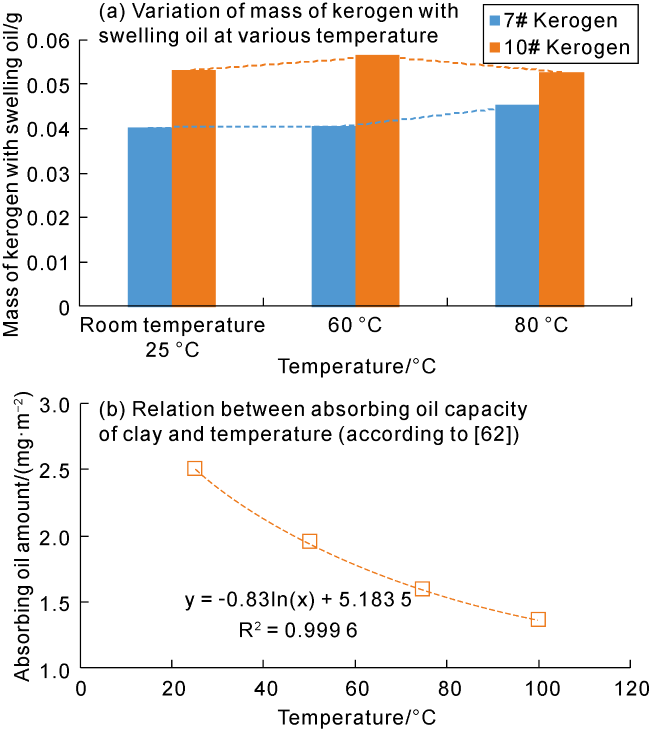
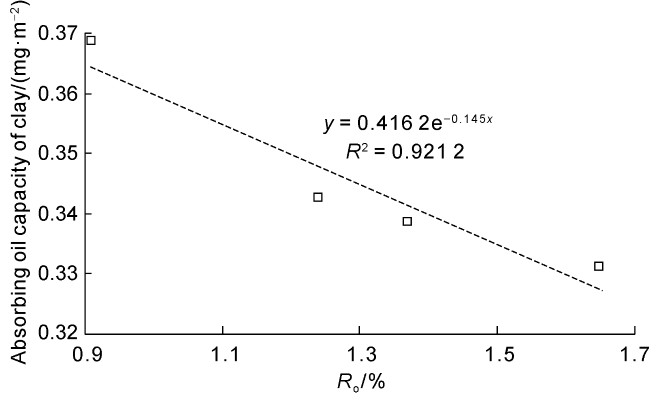
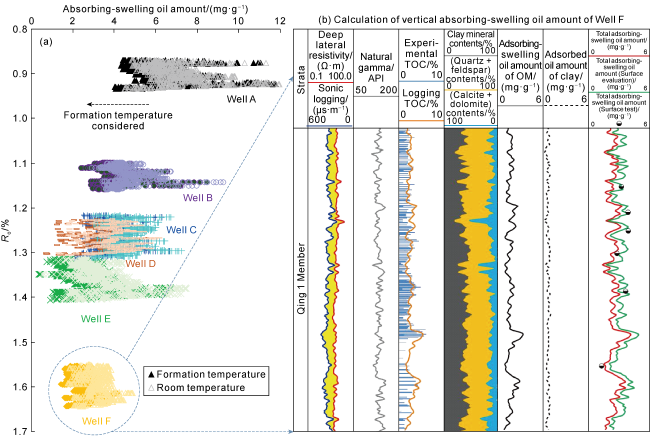
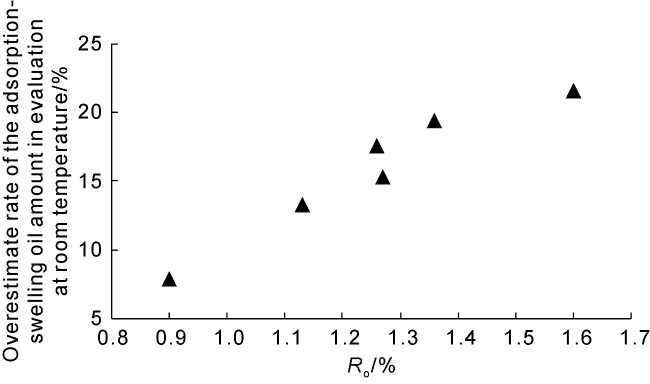
Taking 13 samples in this study as an example, when
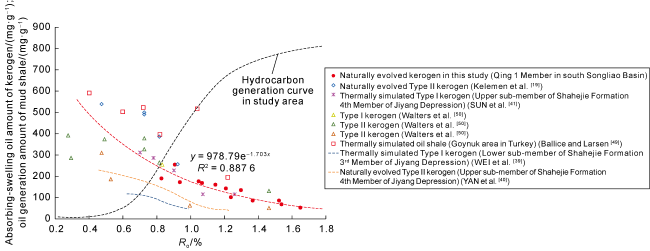
Ro is 0.83%-1.65%, the adsorption-swelling oil capacity (or sorption oil capacity) of kerogen is about 50-250 mg/g. According to the swelling oil experiments of kerogen simulated thermally in gold tubes and that enriched in naturally evolved samples in
Fig. 4, the swelling oil amount of kerogen decreases with increase of
Ro within a wide range. For kerogen
Ro < 0.6%, Ballice and Larsen
[49] carried out swelling experiments of oil shale from Goynuk area of Turkey by thermal simulation to various maturity and derived average
qV of oil shale close to 1.5.Here, the relation between the oil density and
Ro (
Fig. 3) is used to convert them swelling oil amount to 500 mg/g. According to the swelling oil ratio of kerogen with
Ro of 0.47%-0.92% in naturally evolved samples from Kelemen et al.
[19], the swelling oil amount is estimated as about 250-550 mg/g. There are differences in swelling oil amount of kerogen obtained in different study, and the swelling oil amount of kerogen reported by Guangzhou Geochemical Research Institute of Chinese Academy of Sciences also varies. For example, WEI et al.
[39] conducted thermal simulation on kerogen from lower sub-member of Shahejie Formation 3
rd Member of Jiyang Depression in golden tube, and found that the swelling oil amount of kerogen with
Ro of 0.6%-1.0% is about 50-120 mg/g. SUN et al.
[41] proposed that for kerogen in the upper sub-member of Shahejie Formation 4
th Member of Jiyang Depression, when
Ro is 0.7%-1.25%, the swelling oil amount is about 100-300 mg/g, which is close to the results in this study. Beside the maturity, the swelling oil capacity of kerogen is related to its type and thermal evolution. Walters et al.
[50] reported that the swelling ratio of Type II kerogen is higher than that of Type III kerogen.The swelling ratio of kerogen in naturally evolved samples is generally higher than that in thermally simulated samples. We suggest that this is possibly due to the kerogen chemical structure. Geological evolution of kerogen mostly occurs below 200 °C, and thermal simulation is carried out above 300 °C and even at 600 °C. High temperature possibly changes the physicochemical properties of kerogen and causes chemical reaction that does not occur at geologic temperature less than 200 °C
[51]. Secondary cracking of hydrocarbon is more significant in thermal simulation experiment in closed system, and a large amount of coke is generated during coking reaction
[52], which causes more concentrated aromatic ring structure and higher cross-linking density, resulting in the swelling capacity possibly lower than that of naturally evolved sample. According to the measured swelling oil amount of 13 naturally evolved kerogen, the relationship between the swelling oil amount and
Ro was fitted (
Fig. 4).