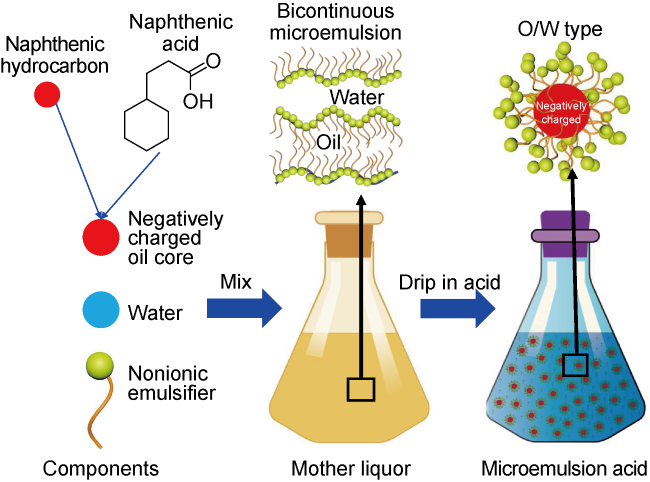
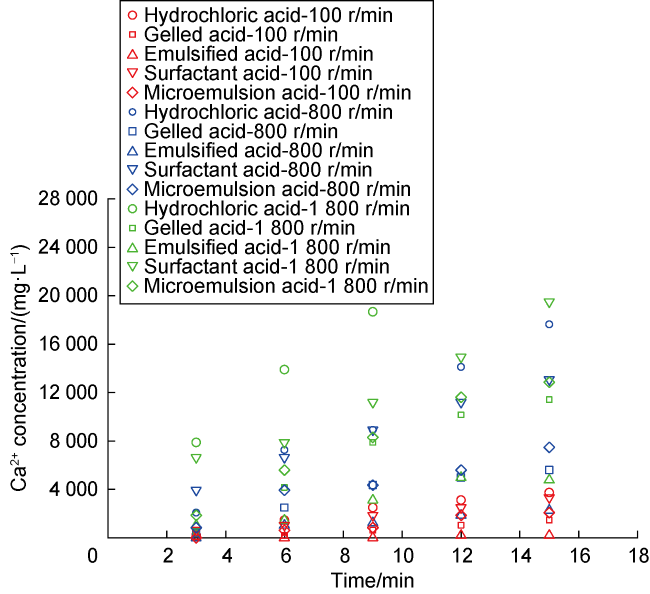
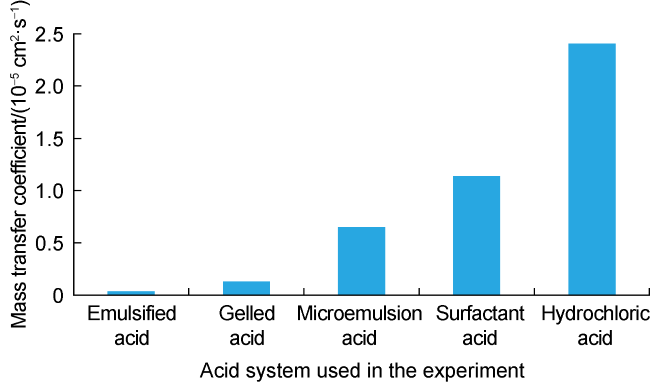
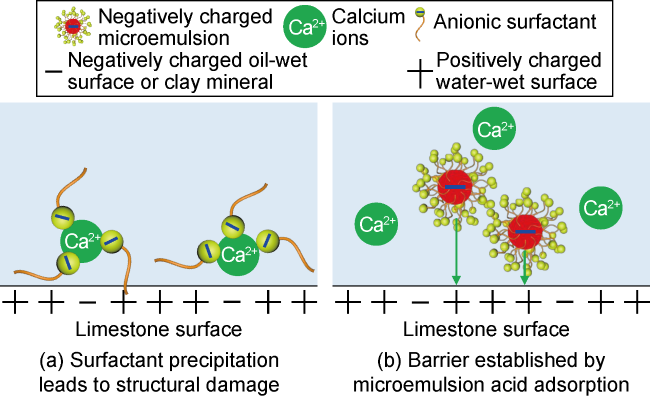
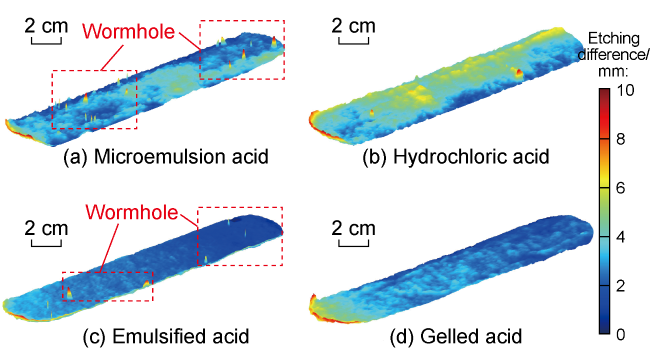
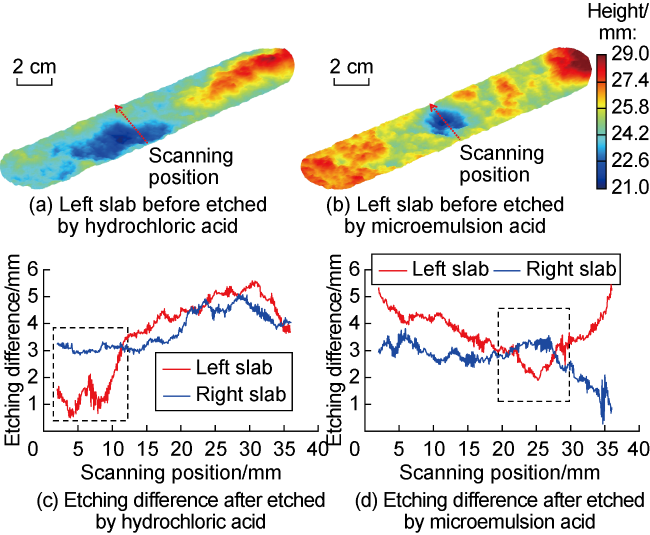
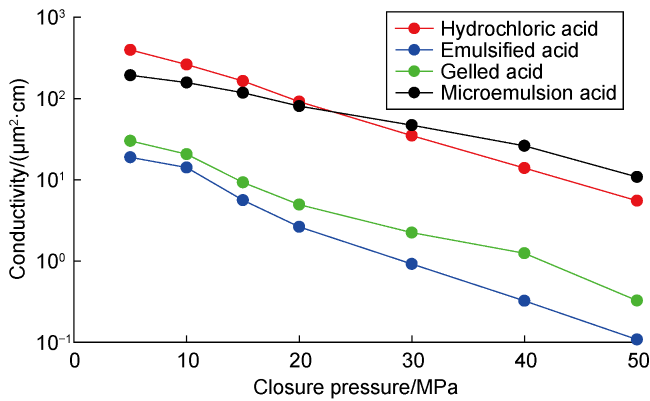
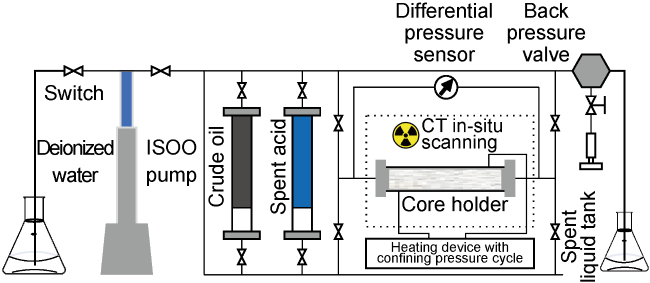
Carbonate reservoirs in the Middle East are dominated by porous bioclastic limestones, which are usually large in scale and less than 3000 m in burial depth
[5]. These reservoirs have three important characteristics
[6]: (1) Natural fractures are not developed or less developed. With strong microscopic heterogeneity and wide range of throat size distribution, the reservoirs are susceptible to solid phase invasion and water lock damage; (2) The purity of calcite minerals is extremely high (generally higher than 95%), and it is extremely difficult to form rough type etchings with the conventional acid fracturing; (3) The rock strength is low, and most of the reservoirs are characterized by plasticity, and the conductivity will decay quickly after acid fracturing stimulation. The conventional acid fracturing treatment based on high viscous acid cannot create fractures with effective etched morphology. In addition, high viscous acid produces a large amount of residue, and the presence of spent acid is easy to induce water lock. Both of them may block oil and gas flow channels
[7]. Therefore, this method isn’t applicable for porous bioclastic limestone. Recent studies have shown that multi-stage fracturing with alternating injection of low-viscosity and retarded acid can significantly improve the stimulation effect of these reservoirs
[1,8]. However, because of commercial confidentiality, the characteristics of acid retardation, acid etching and flowback have not been reported in open literatures. Shen et al.
[9] prepared low-viscosity surfactant micellar acid (surfactant acid) by adding cationic surfactants to hydrochloric acid, and a retardation rate of 71% was achieved by adsorption test on rock surface. The idea of adsorption retardation has been reported several times, suggesting that recovery enhancing agents such as microemulsions and nanoparticles can adsorb onto rock surface and slow down acid-rock reaction
[10⇓-12]. Diluted microemulsion is an oil-in-water nanofluid prepared by diluting bicontinuous microemulsion with water
[13]. It is consistent with the viscosity of the diluted phase, and there is no residue damage. It has the effect of imbibition and displacement of crude oil when added into fracturing fluid
[14]. Diluted microemulsion has been widely used in hydraulic fracturing stimulation
[15], but its suitability for acid fracturing has not been tested due to differences in pH and salinity of the diluted phase.