Introduction
1. Experimental method
1.1. Materials
1.2. Nanofluid preparation
Table 1. Ionic composition, viscosity, and density of fluids [18] |
| Brine | Mass concentration of ions/(mg·L−1) | Density@25 °C/ (g·cm−3) | Viscosity @25 °C/ (mPa·s) | IFT@25 °C/ (mN·m−1) | Salinity/ (mg·L−1) | pH | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| NaCl | CaCl2 | KCl | MgCl2 | Na2SO4 | ||||||
| Formation water | 65 560 | 14 270 | 500 | 4340 | 330 | 1.05 | 1.09 | 11.56 | 85 000 | 6.3 |
| Seawater | 30 850 | 6710 | 240 | 2040 | 160 | 1.03 | 0.97 | 12.40 | 40 000 | 6.3 |
| 10-times diluted sea water | 3090 | 670 | 20 | 200 | 20 | 1.00 | 0.90 | 14.25 | 4000 | 6.4 |
Note: The non-polar crude oil has a density of 0.87 g/cm3 and a viscosity of 17.7 mPa•s at 25 °C. |
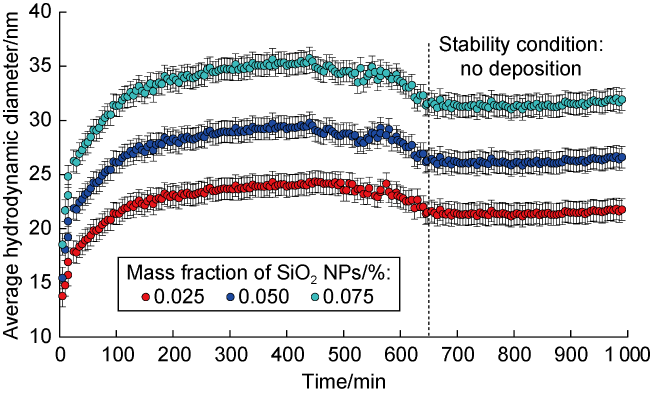
Fig. 1. Average hydrodynamic diameter of SiO2 nanofluids of different mass fractions in formation water. |
1.3. Core flooding experiment
1.4. USBM index and contact angle measurements
1.5. Oil-water IFT measurement
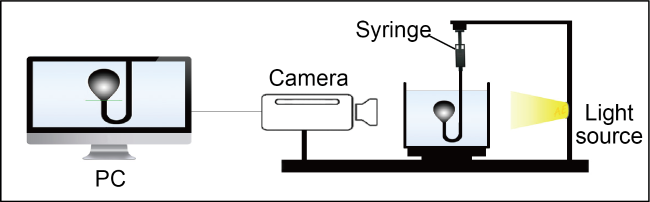
Fig. 2. A schematic of the instrument used to measure the oil-water IFT [20]. |
1.6. Zeta potential measurement
2. Effects of NP mass fraction and brine salinity
2.1. USBM index
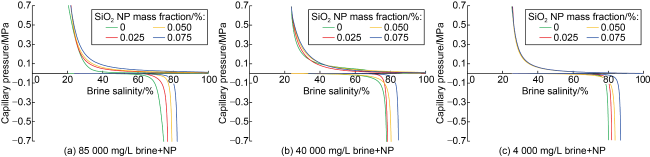
Fig. 3. Effect of NP mass fraction on the capillary pressure curves using three brine salinities and the synthetic Berea core sample. |
Table 2. Effects of NP mass fraction and brine salinity on end-point parameters of capillary pressure curves of synthetic Berea sandstone |
| Brine type | SiO2 NP mass fraction/% | Area under drainage pressure curve/MPa | Area under imbibition pressure curve/MPa | USBM index |
|---|---|---|---|---|
| Formation water | 0 | 3.004 7 | 1.638 1 | 0.263 5 |
| 0.025 | 3.142 5 | 1.509 1 | 0.318 6 | |
| 0.050 | 3.136 3 | 1.221 1 | 0.409 7 | |
| 0.075 | 3.122 1 | 0.930 1 | 0.525 9 | |
| Seawater | 0 | 2.804 8 | 1.347 5 | 0.318 4 |
| 0.025 | 3.878 1 | 1.645 7 | 0.372 3 | |
| 0.050 | 3.830 4 | 1.320 4 | 0.462 6 | |
| 0.075 | 3.875 1 | 0.936 3 | 0.616 9 | |
| 10-times diluted sea water | 0 | 3.007 7 | 1.254 3 | 0.379 8 |
| 0.025 | 3.563 4 | 1.260 6 | 0.451 3 | |
| 0.050 | 3.580 1 | 1.108 6 | 0.509 1 | |
| 0.075 | 3.577 6 | 0.634 0 | 0.751 5 |
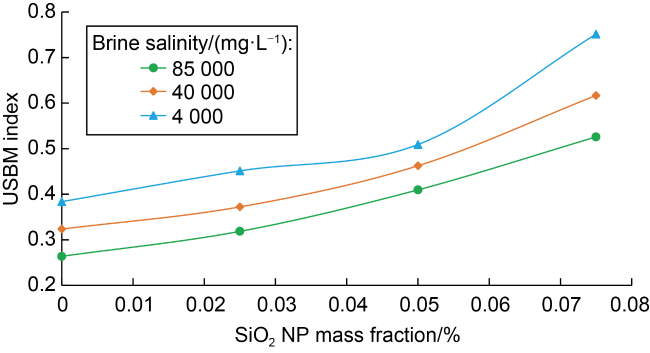
Fig. 4. Effect of NP mass fraction and brine salinity on the USBM index of clay-free synthetic Berea sandstones. |
2.2. Contact angle
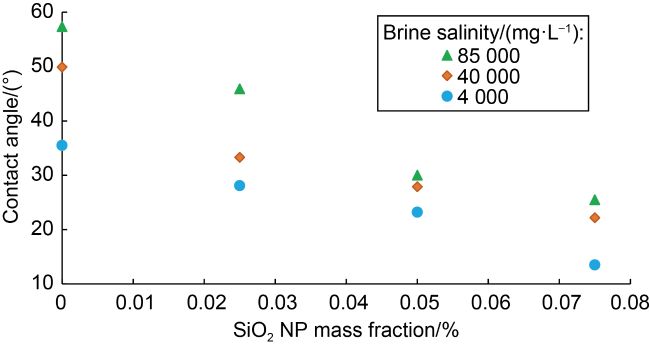
Fig. 5. Effect of NP mass fraction and brine salinity on the contact angle between Berea sandstone and oil. |
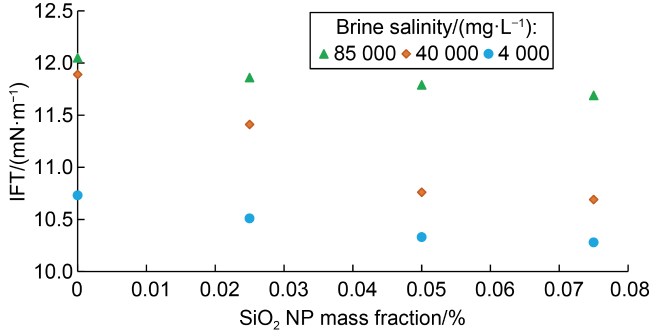
Table 3. Contact angle and IFT measurements for different brine salinities and NP mass fractions |
| Salinity/(mg•L−1) | SiO2 NP mass fraction/% | Average contact angle/(°) | IFT/(mN·m−1) | Relative error in IFT/% |
|---|---|---|---|---|
| 85 000 | 0 | 57.35±1.15 | 12.05 | 0.41 |
| 0.025 | 45.90±1.60 | 11.86 | 0.42 | |
| 0.050 | 30.01±2.75 | 11.79 | 0.42 | |
| 0.075 | 25.50±0.97 | 11.69 | 0.43 | |
| 40 000 | 0 | 49.90±1.27 | 11.89 | 0.42 |
| 0.025 | 33.30±0.47 | 11.41 | 0.44 | |
| 0.050 | 27.90±0.65 | 10.76 | 0.46 | |
| 0.075 | 22.20±0.30 | 10.69 | 0.47 | |
| 4000 | 0 | 35.50±0.41 | 10.73 | 0.47 |
| 0.025 | 28.10±0.30 | 10.51 | 0.48 | |
| 0.050 | 23.20±0.58 | 10.33 | 0.48 | |
| 0.075 | 13.50±1.65 | 10.28 | 0.49 |
2.3. Oil-water IFT
Fig. 6. Effects of NP mass fraction and brine salinity on oil-water IFT. |
2.4. Nanofluid rheology
2.5. Nanofluid stability
Table 4. Zeta potential measurements for nanosuspensions |
| Salinity/(mg•L−1) | SiO2 NP mass fraction/% | Zeta potential/mV |
|---|---|---|
| 0.025 | −6.50 | |
| 85 000 | 0.050 | −2.56 |
| 0.075 | −4.45 | |
| 0.025 | −15.70 | |
| 40 000 | 0.050 | −12.80 |
| 0.075 | −12.40 | |
| 0.025 | −30.30 | |
| 4000 | 0.050 | −27.50 |
| 0.075 | −28.90 |
2.6. NP retention
Table 5. Effect of NP mass fraction on rock permeability |
| NP mass fraction/ % | Brine salinity/ (mg•L−1) | Initial permeability/ 10−3 μm2 | Permeability After nanofluid flooding/ 10−3 μm2 | Permeability After brine flooding/ 10−3 μm2 |
|---|---|---|---|---|
| 0.025 | 85 000 | 53.397 | 48.643 | 51.205 |
| 40 000 | 64.866 | 47.218 | 59.810 | |
| 4000 | 67.406 | 51.035 | 65.000 | |
| 0.050 | 85 000 | 52.535 | 41.404 | 50.552 |
| 40 000 | 65.300 | 43.772 | 62.453 | |
| 4000 | 68.268 | 45.431 | 64.916 | |
| 0.075 | 85 000 | 52.987 | 28.079 | 47.822 |
| 40 000 | 65.108 | 35.369 | 58.724 | |
| 4000 | 67.449 | 37.870 | 61.430 |
Table 6. Relative change of absolute permeability of synthetic Berea core samples |
| Experiment | Porosity/ % | Average pore radius/mm | Initial permeability/ 10−3 μm2 | Relative change after nanofluid flooding/% | Relative change after brine flooding/% |
|---|---|---|---|---|---|
| 85 000 mg/L Brine+0.025% SiO2 | 19.8 | 0.52 | 48.6 | 8.9 | 4.1 |
| 85 000 mg/L Brine+0.050% SiO2 | 19.3 | 0.58 | 41.4 | 21.2 | 3.8 |
| 85 000mg/L Brine+0.075% SiO2 | 19.5 | 0.59 | 28.1 | 47.0 | 9.8 |
| 40 000 mg/L Brine+0.025% SiO2 | 19.6 | 0.52 | 47.2 | 27.2 | 7.8 |
| 40 000 mg/L Brine+0.050% SiO2 | 19.1 | 0.58 | 43.8 | 33.0 | 4.4 |
| 40 000 mg/L Brine+0.075% SiO2 | 19.3 | 0.59 | 35.4 | 45.7 | 9.8 |
| 4000 mg/L Brine+0.025% SiO2 | 19.6 | 0.52 | 51.0 | 24.3 | 3.6 |
| 4000 mg/L Brine+0.050% SiO2 | 19.2 | 0.58 | 45.4 | 33.5 | 4.9 |
| 4000 mg/L Brine+0.075% SiO2 | 19.3 | 0.59 | 37.9 | 43.9 | 8.9 |
2.7. Oil recovery
Table 7. Recovery factor and related parameters obtained from capillary pressure measurements |
| Salinity/ (mg·L−1) | SiO2 mass fraction/% | Irreducible water saturation/% | Remaining oil saturation/% | Recovery factor/% |
|---|---|---|---|---|
| 85 000 | 0 | 21 | 25 | 68.9 |
| 0.025 | 21 | 23 | 71.0 | |
| 0.050 | 21 | 21 | 73.9 | |
| 0.075 | 21 | 18 | 77.8 | |
| 40 000 | 0 | 24 | 22 | 70.5 |
| 0.025 | 24 | 21 | 72.4 | |
| 0.050 | 24 | 19 | 74.4 | |
| 0.075 | 24 | 15 | 79.7 | |
| 4 000 | 0 | 26 | 20 | 72.8 |
| 0.025 | 26 | 18 | 75.5 | |
| 0.050 | 25 | 16 | 78.2 | |
| 0.075 | 26 | 13 | 82.3 |