Introduction
1. Materials and methods
1.1. Crude oil and rock samples
Table 1. Relevant crude oil properties |
| Parameter | Value | Parameter | Value |
|---|---|---|---|
| Saturates content | 42.30% | TAN | 0.23 mg/g |
| Aromatics content | 26.90% | TBN | 1.90 mg/g |
| Resin content | 19.70% | Relative density | 0.920 6 |
| Asphaltene content | 11.12% | Density at 114 °C and 27.6 MPa | 876.9 kg/m3 |
| Sulfur content | 2.53% |
Table 2. Metal content in oil samples |
| Metal | Content/(mg·kg−1) | Metal | Content/(mg·kg−1) |
|---|---|---|---|
| Ba | <0.50 | Na | 23.84 |
| Ca | 6.34 | Sr | 0.35 |
| Fe | 2.35 | Ni | 20.78 |
| Mg | 0.17 | V | 101.00 |
| K | 0.64 |
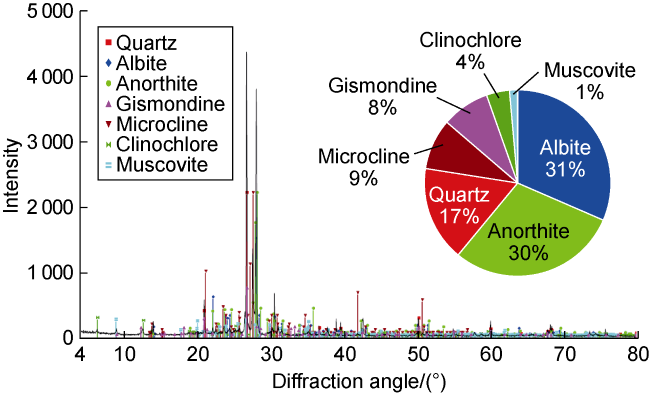
Fig. 1. Mineral composition of the core. |
1.2. Brines
Table 3. Brine characteristic parameters |
| Brine | pH | Conductivity/ (mS·cm−1) | Total dissolved solids/(mg·L−1) | Total hardness as CaCO3/(mg·L−1) | Salinity as NaCl/ (mg·L−1) | Density at 114 °C and 27.6 MPa/(kg·m−3) |
|---|---|---|---|---|---|---|
| FW | 8.03 | 131.63 | 101 208.57 | 9766.67 | 94 000 | 1027.14 |
| SW | 8.03 | 54.93 | 39 362.40 | 6600.00 | 35 000 | 987.29 |
| LSW10% | 7.32 | 6.38 | 3802.82 | 639.33 | 3600 | 964.18 |
1.3. Surfactant
1.4. Interfacial tension (IFT)
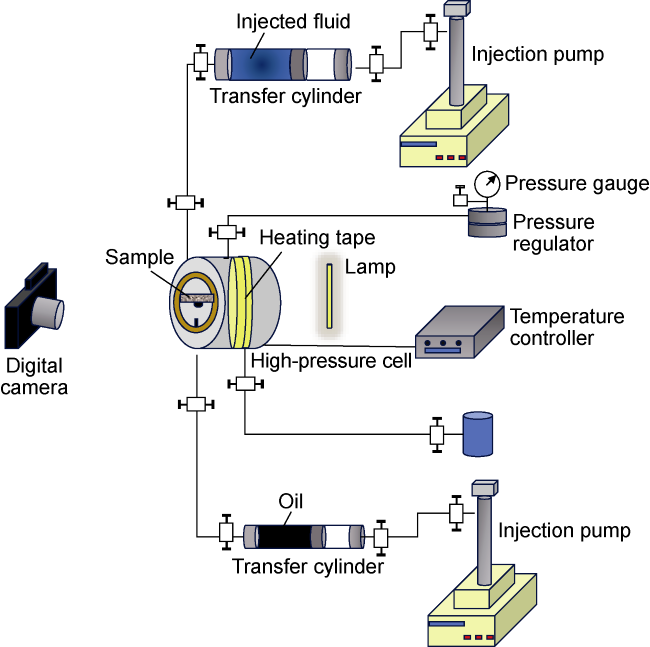
Fig. 2. Schematic of the IFT and contact angle measurement system. |
1.5. Contact angle measurement
1.6. Evaluation of ions
1.7. Carbon chromatography of crude oil
1.8. pH
1.9. Core-flooding experiment
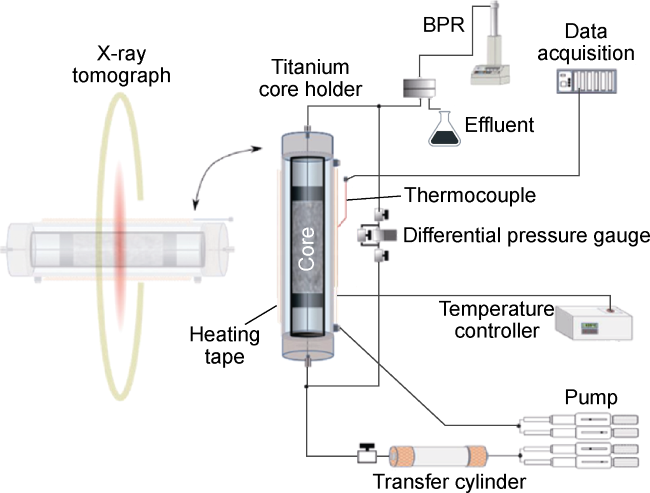
Fig. 3. Schematic of the core-flooding system. |
Table 4. Core parameters |
| Parameter | Value | Parameter | Value |
|---|---|---|---|
| Length | 12.5 cm | Porosity | 24.8% |
| Diameter | 5.0 cm | Permeability | 94.5×10−3 μm2 |
| Dry weight | 531.6 g | Initial oil saturation | 47.5% |
| Pore volume | 61.6 cm3 |
1.10. Tomographic analysis
2. Results and discussion
2.1. Interfacial tension (IFT)
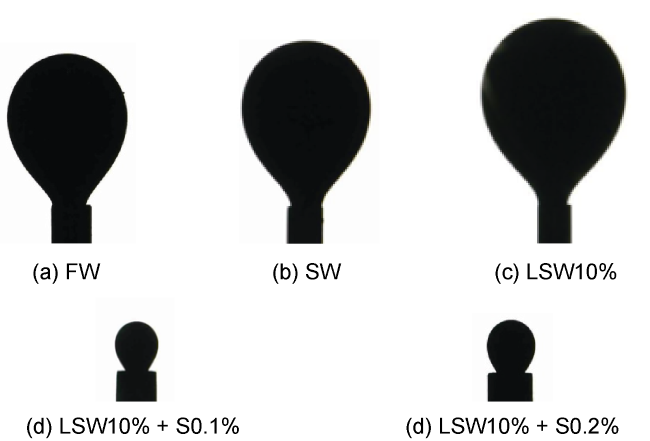
Fig. 4. Evaluation of the IFT between brines and oil. |
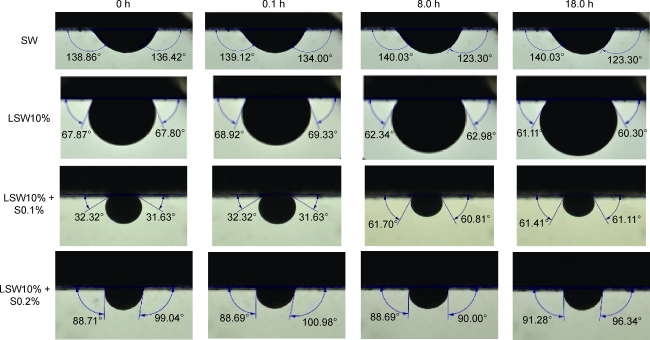
2.2. Contact angle
Fig. 5. Evaluation of the contact angle with different brines. |
2.3. Core-flooding experiment
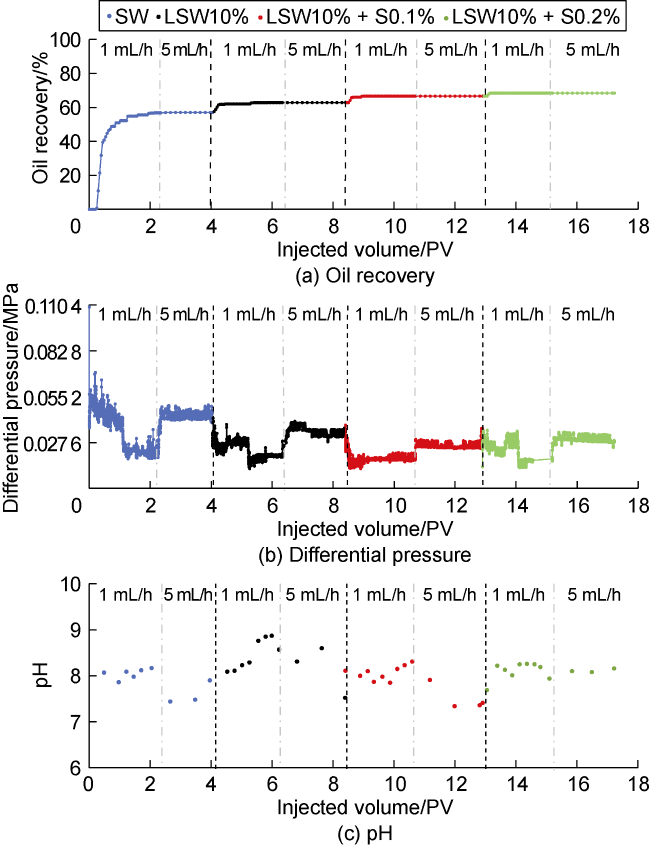
Fig. 6. Core-flooding experiments with different brines. |
2.3.1. Oil recovery
2.3.2. Differential pressure
2.3.3. Effluent pH
2.4. Effluent ion analysis
Table 5. Ion concentrations |
| Brine | Concentration/(mg•L-1) | ||||||
|---|---|---|---|---|---|---|---|
| Ca2+ | Mg2+ | Mn2+ | Na+ | Sr2+ | Ba2+ | Fe2+ | |
| SW in | 382 | 1283 | <0.05 | 9308 | 8.0 | <0.20 | <0.10 |
| SW ef | 874 | 1270 | 5.39 | 11 147 | 9.0 | 0.53 | <0.04 |
| LSW10% in | 43 | 137 | <0.02 | 1095 | 0.8 | <0.20 | <0.04 |
| LSW10% ef | 204 | 108 | 0.66 | 1183 | 1.4 | 0.56 | <0.04 |
| LSW10%+S0.1% in | 43 | 142 | 0.02 | 1215 | 0.8 | <0.20 | <0.89 |
| LSW10%+S0.1% ef | 257 | 68 | 0.82 | 1205 | 1.4 | 0.37 | <0.04 |
| LSW10%+S0.2% in | 43 | 137 | <0.02 | 1279 | 0.8 | <0.20 | <0.20 |
| LSW10%+S0.2% ef | 216 | 83 | 0.89 | 1287 | 1.3 | 0.34 | 0.11 |
Note: in= influent, ef= effluent |
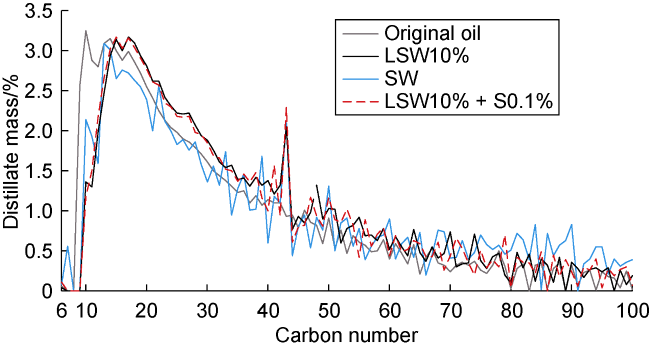
2.5. Chromatographic analysis of crude oil
Fig. 7. Analysis of the oil fractions collected after core- flooding by fractional distillation chromatography. |
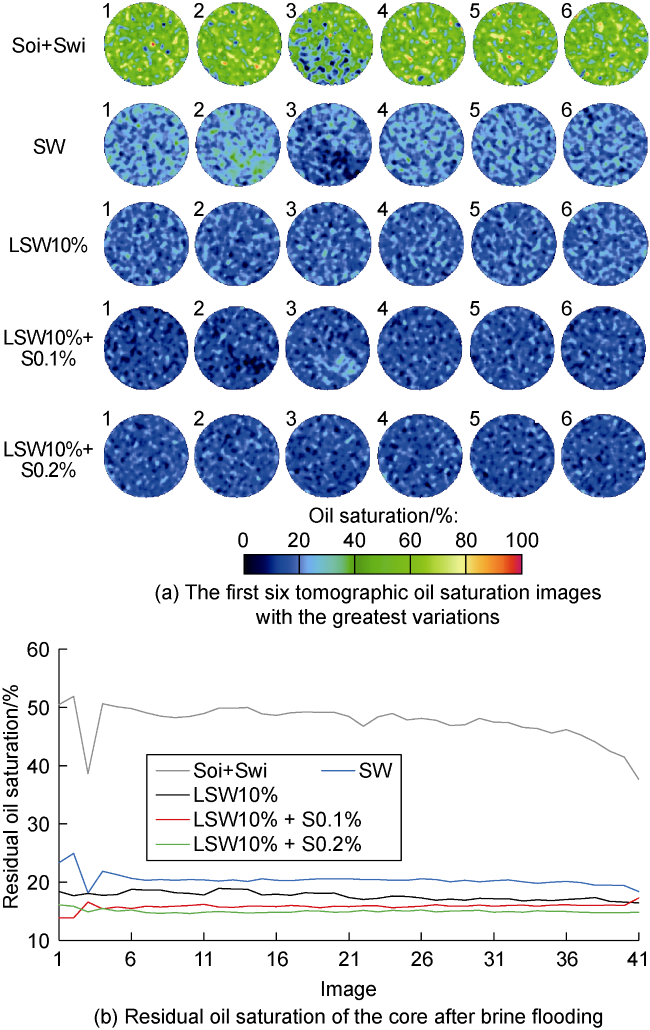
2.6. Tomographic analysis
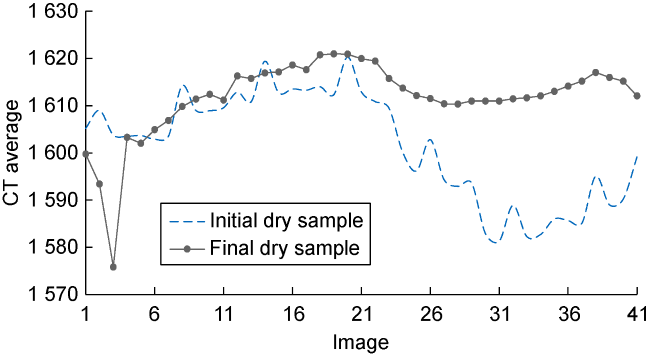
2.6.1. Evaluation of residual oil saturation
Fig. 8. CT distribution of initial dry samples and final dry samples after core-flooding and washing. |
Fig. 9. Oil saturation at the end of injection stages. |
Fig. 10. Sweep efficiency by stages. |
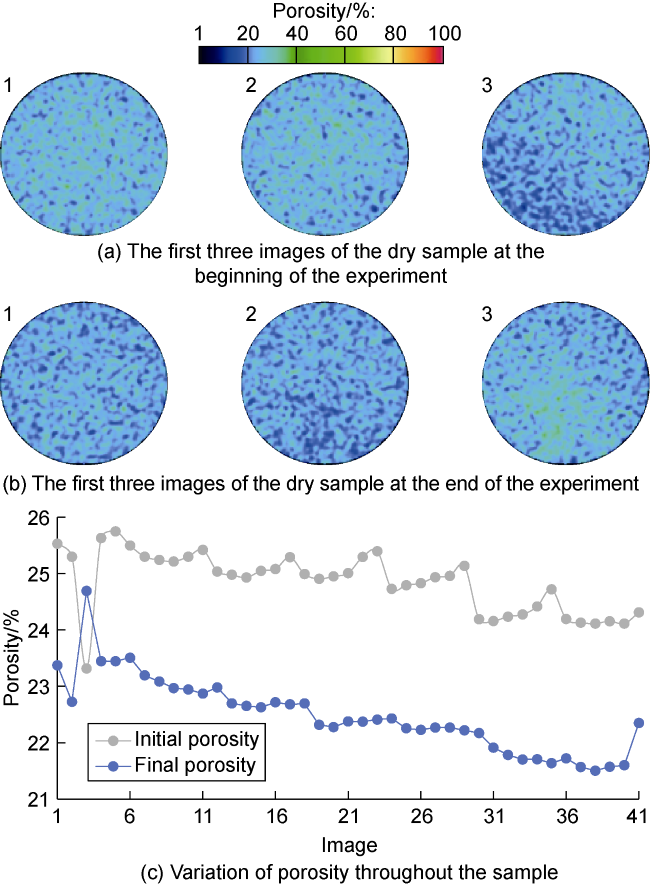
2.6.2. Porosity analysis
Fig. 11. Porosity analysis. |