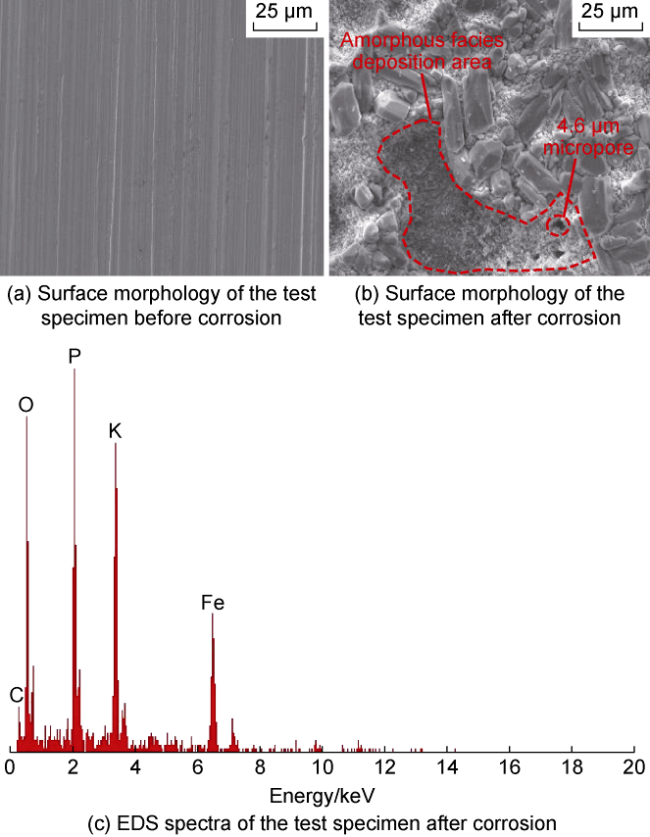
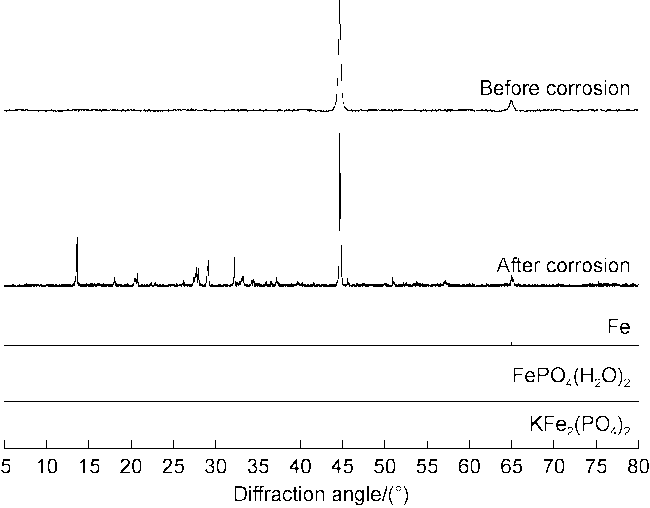
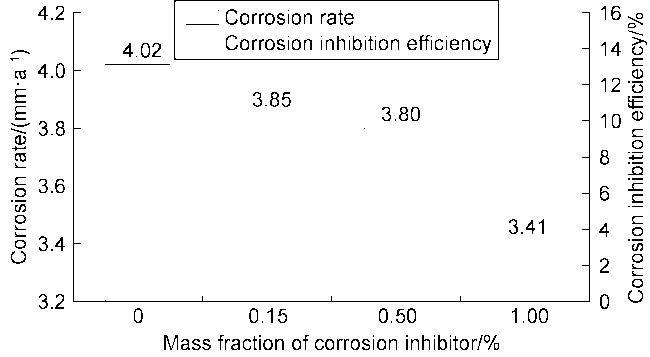
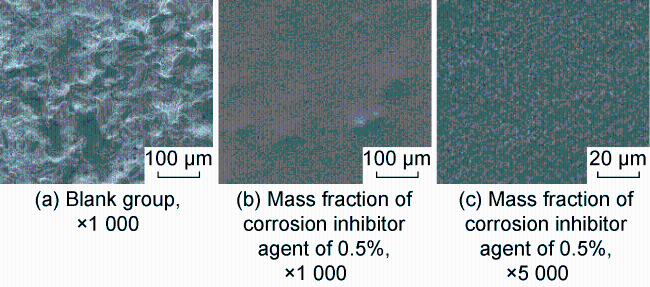
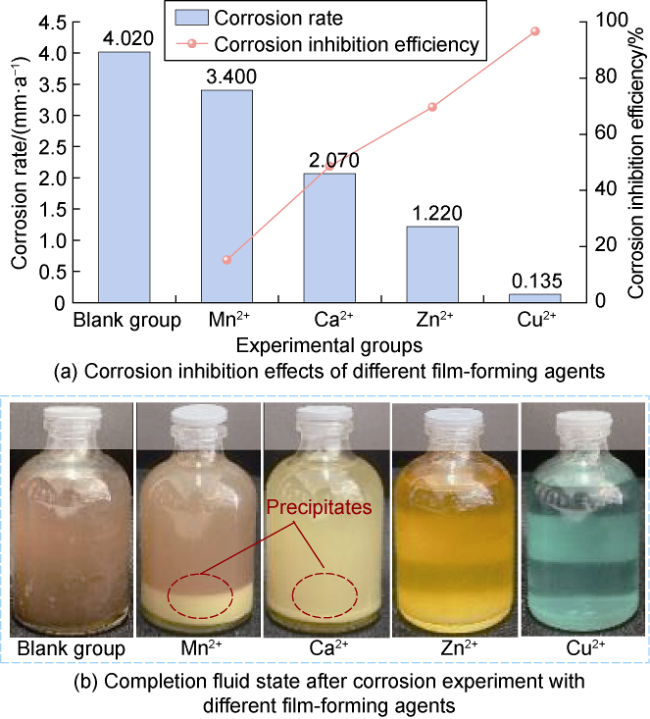
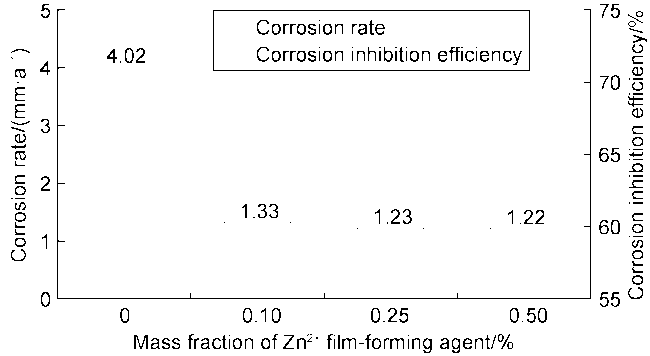
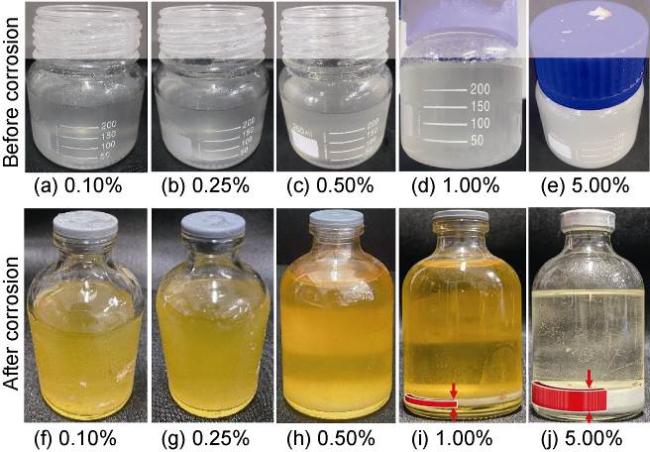
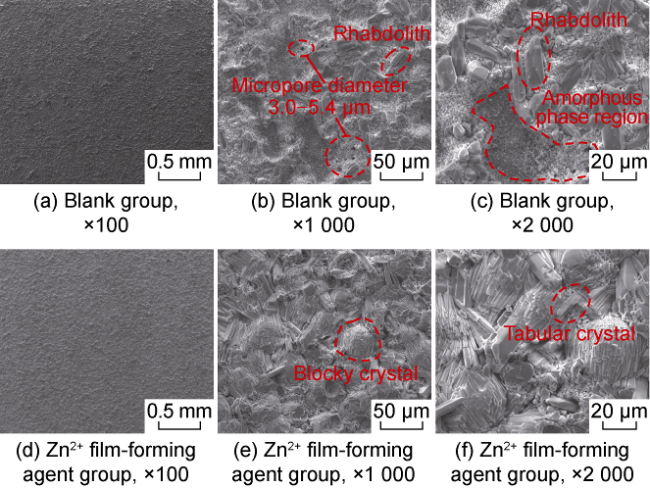
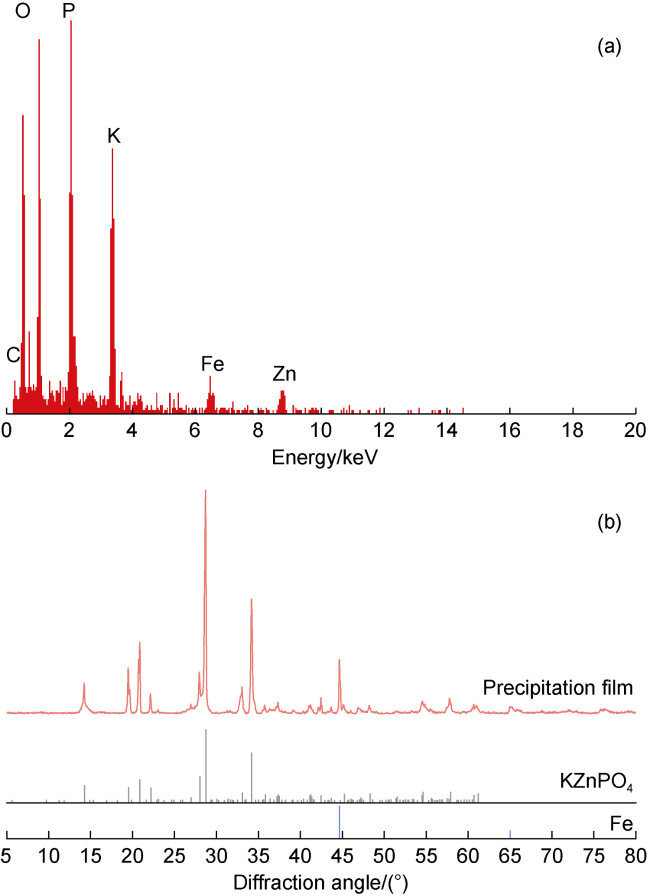
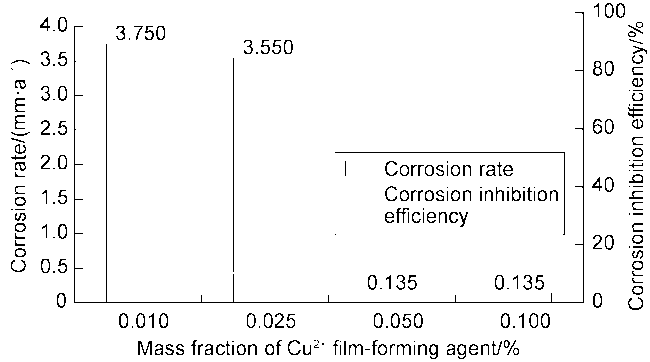
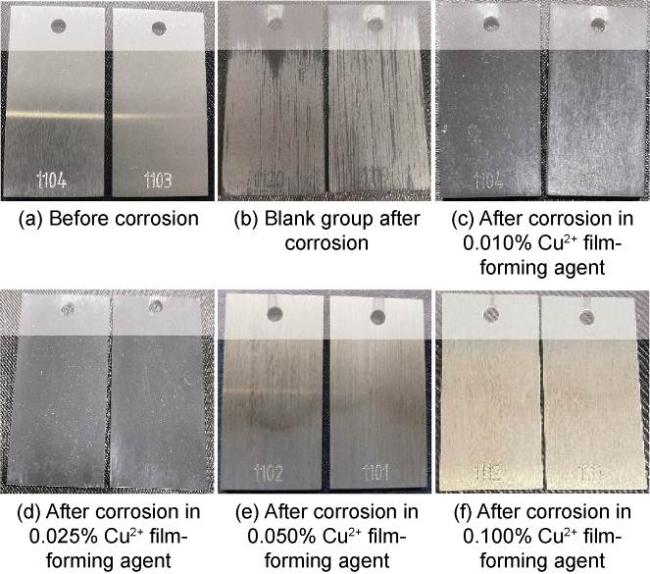
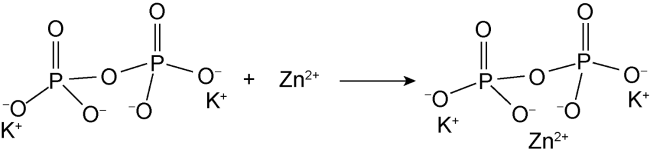High temperature and high pressure oil and gas reservoirs are widely distributed in the East China Sea, the South China Sea, Tarim Basin, NW China, etc.
[1-2] where the formation temperatures are 160-210 °C and the pressure coefficient is 1.5-2.1, which makes the completion of oil and gas wells face severe challenges. Among them, the technology of solid-free completion fluid with low corrosion and high density is lacking. Completion fluid can balance formation pressure, protect reservoir, clean borehole, and control filtration and leakage
[3-4] in the completion process. Clean and solid-free brine completion fluid is widely used in many oil fields all over the world because of its advantages such as adjustable density, environmentally friendly, low reservoir damage, and no water lock effect
[5-6]. However, in deep and ultra-deep wells in China, the commonly used brine completion fluids (i.e., NaCl, KCl, CaCl
2, Ca(NO
3)
2) can no longer meet the needs of actual working conditions
[7-8]. In addition, high-density bromine and formate completion fluids are corrosive, easy to crystallize, and expensive
[9⇓-11]. Jia et al.
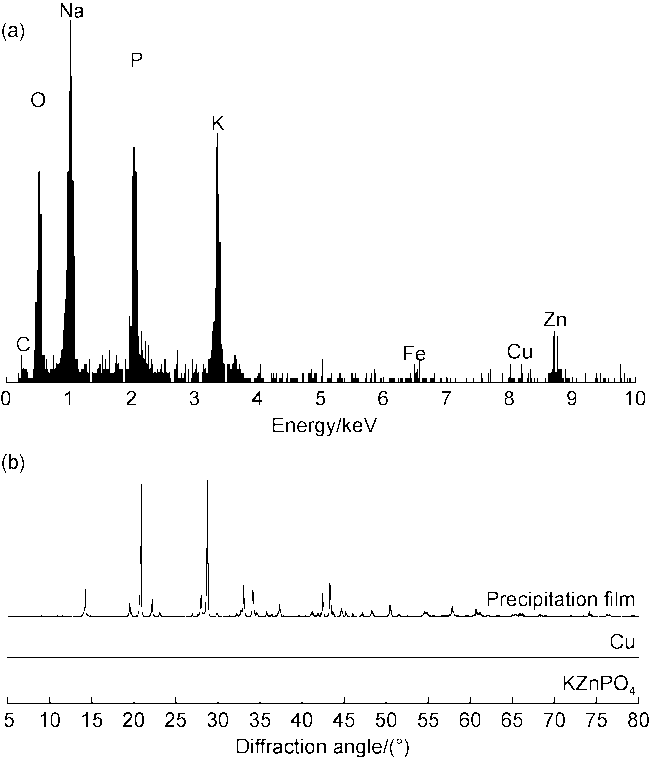
[3] developed a phosphate completion fluid with a maximum density of 1.815 g/cm
3, which can be applied at 180 °C. The phosphate completion fluid has high density, low cost, low toxicity, low reservoir damage and good formation compatibility, and can work in high-temperature and high-pressure oil reservoirs. However, its corrosion mechanism on oil casing at high temperature remains unclear. The effective corrosion control method has not yet been developed.