Introduction
1. Geological setting
Fig. 1 Location of the study area (a), sedimentary facies distribution (b), and provenance-based division and sampling wells distribution of sedimentary area (c) of the Chang 7 Member in the Ordos Basin (modified from Reference [26]). |
2. Samples and methods
3. Petrology of Chang 7 sandstone
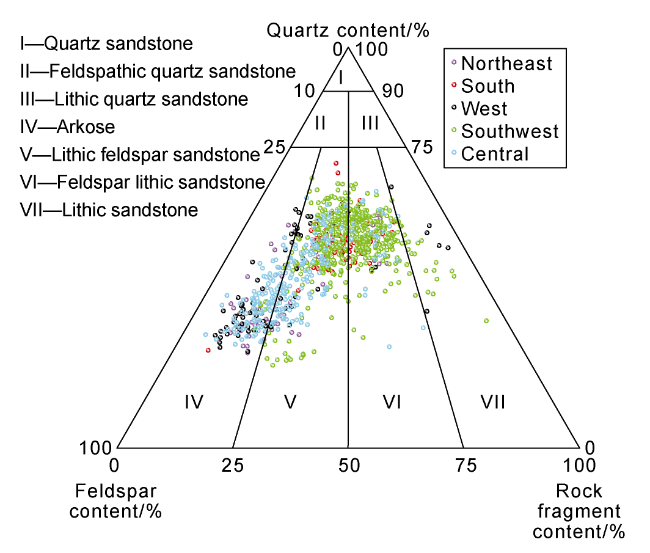
Fig. 2 Ternary diagram of rock classification for the Chang 7 sandstone. |
Table 1 Rock composition and porosity from thin sections of Chang 7 sandstone in the study area |
| Region | Number of samples | Detrital composition/% | Interstitial material/% | Thin section porosity/% | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Quartz | Feldspar | Lithics | Mica | Matrix | Cement | Feldspar dissolution pore | Lithic dissolution pore | Cement dissolution pore | Intergranular pore | Total thin section porosity | ||
| Northeast | 99 | 15.80-51.50/ 25.11 | 12.00-53.50/ 42.52 | 7.00-27.20/ 12.01 | 7.10 | 6.64 | 6.62 | 0.80 | 0.10 | 1.10 | 2.30 | |
| Central | 145 | 11.00-56.50/ 25.10 | 7.00-57.53/ 35.10 | 7.00-41.01/ 14.01 | 7.67 | 10.21 | 7.92 | 0.68 | 0.10 | 1.10 | 2.00 | |
| South | 55 | 21.00-54.00/ 39.10 | 8.00-60.00/ 24.10 | 9.00-24.80/ 17.12 | 4.70 | 10.10 | 4.90 | 1.04 | 0.10 | 0.30 | 1.60 | |
| West | 65 | 16.00-70.00/ 33.08 | 7.30-57.00/ 24.22 | 7.20-44.00/ 24.32 | 4.56 | 8.15 | 5.67 | 1.01 | 0.30 | 0.20 | 3.30 | 4.90 |
| Southwest | 568 | 15.80-51.50/ 40.16 | 12.00-53.50/ 19.08 | 8.10-27.20/ 20.88 | 4.31 | 9.68 | 5.89 | 0.96 | 0.10 | 0.10 | 0.70 | 2.00 |
| Average | 36.45 | 24.47 | 18.94 | 5.14 | 9.39 | 6.21 | 0.91 | 0.10 | 0.08 | 0.95 | 2.19 | |
Note: The value after "/" is average. |
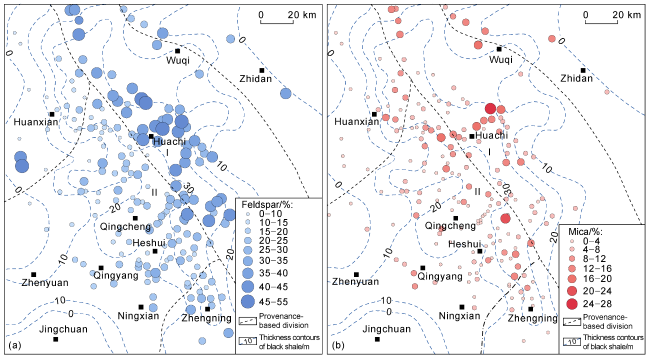
Fig. 3 Distribution of feldspar (a) and mica (b) in the Chang 7 sandstone in the study area (from thin section data). |
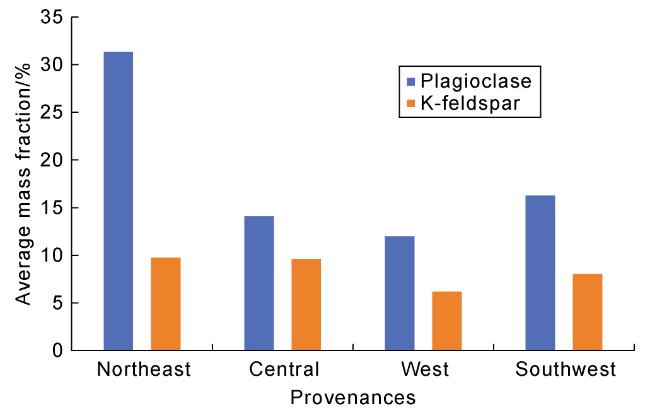
Fig. 4 The average content histogram of plagioclase and K-feldspar in the Chang 7 sandstone in the study area. |
4. Diagenetic processes of feldspar in Chang 7 sandstone
4.1. Feldspar overgrowth and replacement by authigenic mineral
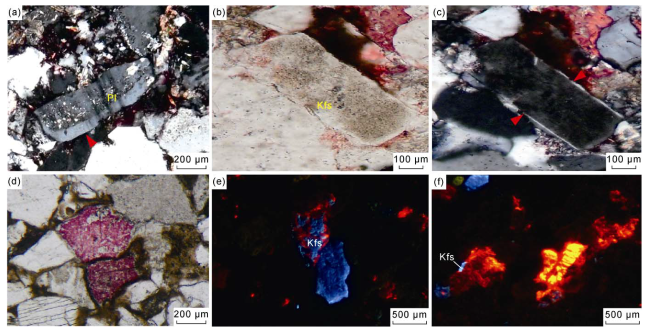
Fig. 5 Feldspar overgrowth and replacement. (a) Sericitized plagioclase with overgrowth; 1 216.53 m; Well Zheng 9; cross-polarized light (XPL); (b) Feldspar overgrowth; 1 178.50 m; Well Zheng 8; plane-polarized light (PPL); (c) Kaolinized K-feldspar with slightly dissolved edge; 1 178.50 m; Well Zheng 8; XPL; (d) K-feldspar completely dissolved, a small amount of residue, and filled with calcite; PPL; 2 201.60 m; Well Hu 218; (e) K-feldspar locally replaced by calcite; 2 647.83 m; Well Huan 54; cathodoluminescence; (f) K-feldspar replaced by early calcite; 1 634.70 m; Well Ning 29; cathodoluminescence. Pl—plagioclase; Kfs—K-feldspar. |
4.2. Feldspar dissolution
4.2.1. Feldspar dissolution pores
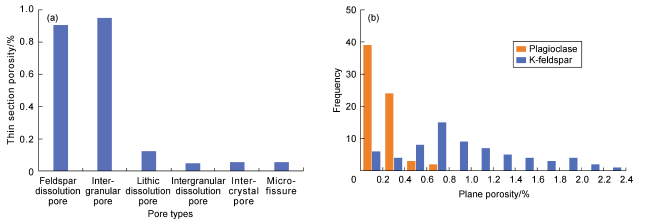
Fig. 6 Thin section porosity of different pores (a) and the dissolution porosity of plagioclase and K-feldspar (b) in Chang 7 sandstone. |
Fig. 7 Photomicrographs of feldspar dissolution in the Chang 7 sandstone in the study area. (a) K-feldspar dissolution pores filled with red resin; 1 973.79 m; Well Xi 23; PPL; (b) Residual K-feldspar skeleton and undissolved adjacent microcline; 1 973.79 m; Well Xi 23; PPL; (c) Plagioclase ( probably albite ) partially dissolved and dissolution pores filled with hydrocarbons; 1 973.79 m; Well Xi 23; PPL; (d) The same field of view as Fig. a; XPL; (e) Microcline characterized by crossed twinning; the same field of view as Fig. b; XPL; (f) Albite characterized by polysynthetic twin; the same field of view as Fig. c; XPL; (g) Residual K-feldspar skeleton and adjacent dissolved kaolinized K-feldspar; 1 689.33 m; Well Ning 28; PPL; (h) The same field of view as Fig. g; XPL; (i) Undissolved kaolinized K-feldspar; 1 689.33 m; Well Ning 28; PPL (left ) and XPL (right ). Ab—albite; Mic—microcline. |
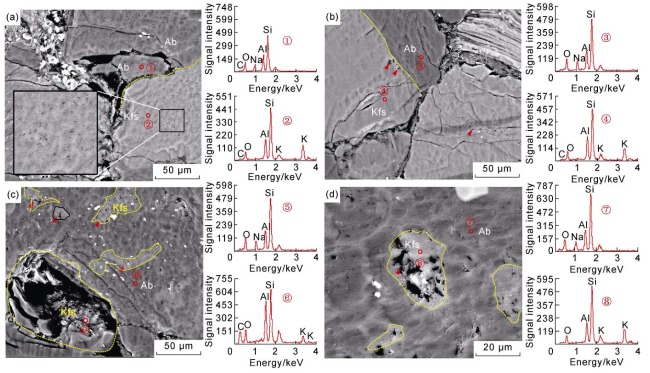
Fig. 8 SEM images and energy spectra of feldspar in Chang 7 sandstone (①-⑧ are energy spectrum detecting points). (a) K-feldspar (light gray) has more micropores than adjacent albite (gray); 1 944.9 m; Well Shan 110; (b) K-feldspar and albite contact in twinning; K-feldspar has dissolution pores with different sizes along the crystal boundary, but no pores found in albite adjacent to biotite; 1 944.9 m; Well Shan 110; (c) pores in albite is closely related to K-feldspar solid solution; 1 498.6 m; Well Shan 110; (d) micropores in K-feldspar are more than in host albite mineral; 1 498.6 m; Well Shan 110. |
4.2.2. Dissolution process of feldspar
Fig. 9 SEM images of feldspar dissolution pores and by-product minerals in Chang 7 sandstone. (a) Dissolution pores filled with authigenic quartz and illite; 2 110.00 m; Well Li 150; ( b ) K-feldspar dissolution pores filled with quartz; 2 422.54 m; Well An 72; (c) K-feldspar dissolution pore, and authigenic kaolinite deposits in intergranular pores; 2 300.39 m; Well Hu 211; (d) pores partially filled with albite and calcite; 1 809.40 m; Well Zhuang 144; (e) authigenic albite and illite deposited in dissolution pores; 2 572.00 m; Well Geng 170; (f) short-plate authigenic albite and illite in dissolution pores; 2 463.04 m; Well Huan 87. Cal—calcite; I—illite; K—kaolinite; Qtz—quartz. |
4.3. Regional distribution of feldspar dissolution porosity
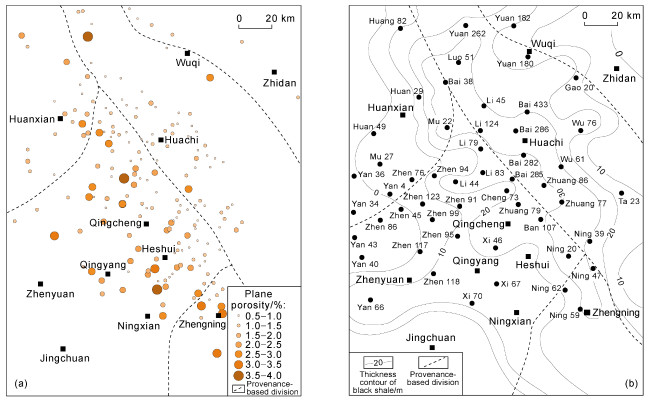
Fig. 10 Thin section porosity distribution of feldspar dissolution pores (a) and thickness of dark mudstone (b) in Chang 7 Member. |
4.4. Dissolution experiment of feldspar
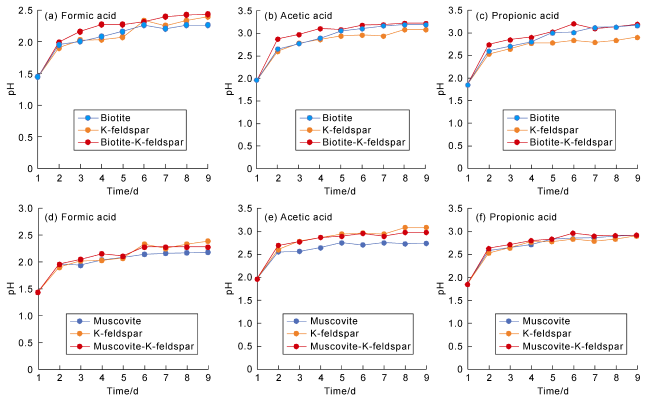
Table 2 The pH variation of solution groups with different mineral assemblages |
| Mineral | Organic acids | pH | Hydrogen ion concentration/(10−3 mol·L−1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 8 | Day 9 | |||
| Biotite | Formic acid | 1.44 | 1.95 | 2.00 | 2.08 | 2.16 | 2.26 | 2.20 | 2.26 | 2.26 | 30.81 |
| Acetic acid | 1.96 | 2.65 | 2.77 | 2.89 | 3.05 | 3.10 | 3.16 | 3.19 | 3.19 | 10.32 | |
| Propionic acid | 1.85 | 2.60 | 2.70 | 2.80 | 3.00 | 3.01 | 3.12 | 3.13 | 3.16 | 13.41 | |
| Muscovite | Formic acid | 1.44 | 1.96 | 1.94 | 2.04 | 2.08 | 2.14 | 2.16 | 2.17 | 2.18 | 29.70 |
| Acetic acid | 1.96 | 2.55 | 2.56 | 2.64 | 2.75 | 2.70 | 2.75 | 2.73 | 2.74 | 9.15 | |
| Propionic acid | 1.85 | 2.58 | 2.65 | 2.71 | 2.83 | 2.85 | 2.86 | 2.89 | 2.92 | 12.88 | |
| K-feldspar | Formic acid | 1.44 | 1.90 | 2.02 | 2.03 | 2.07 | 2.33 | 2.25 | 2.33 | 2.39 | 28.43 |
| Acetic acid | 1.96 | 2.60 | 2.78 | 2.86 | 2.94 | 2.96 | 2.94 | 3.08 | 3.08 | 10.07 | |
| Propionic acid | 1.85 | 2.53 | 2.64 | 2.77 | 2.78 | 2.83 | 2.79 | 2.83 | 2.90 | 12.69 | |
| Biotite + K-feldspar | Formic acid | 1.44 | 1.99 | 2.16 | 2.27 | 2.27 | 2.31 | 2.39 | 2.42 | 2.43 | 32.13 |
| Acetic acid | 1.96 | 2.87 | 2.97 | 3.10 | 3.08 | 3.18 | 3.19 | 3.22 | 3.22 | 10.19 | |
| Propionic acid | 1.85 | 2.74 | 2.85 | 2.90 | 3.02 | 3.20 | 3.09 | 3.13 | 3.19 | 13.20 | |
| Muscovite + K-feldspar | Formic acid | 1.44 | 1.96 | 2.05 | 2.15 | 2.11 | 2.28 | 2.28 | 2.28 | 2.28 | 28.15 |
| Acetic acid | 1.96 | 2.69 | 2.77 | 2.86 | 2.89 | 2.95 | 2.89 | 2.97 | 2.97 | 9.71 | |
| Propionic acid | 1.85 | 2.63 | 2.71 | 2.80 | 2.83 | 2.96 | 2.90 | 2.91 | 2.92 | 12.74 | |
Fig. 11 The pH variation curves of reaction solutions of organic acids with different mineral assemblages. |
5. Controlling factors on feldspar dissolution
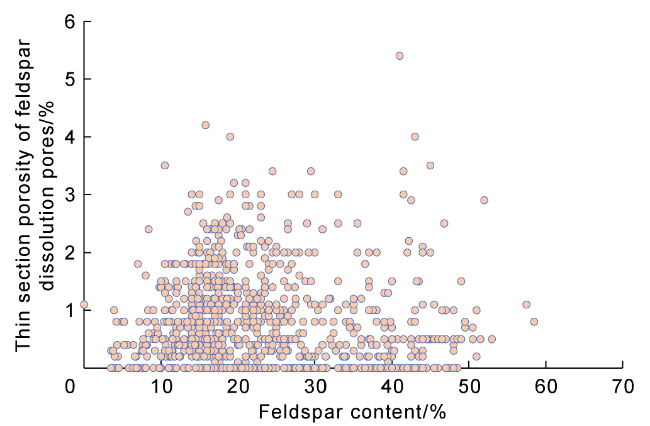
5.1. Feldspar content and type
Fig. 12 The scatter plots of feldspar content and feldspar dissolution porosity in the study area. |