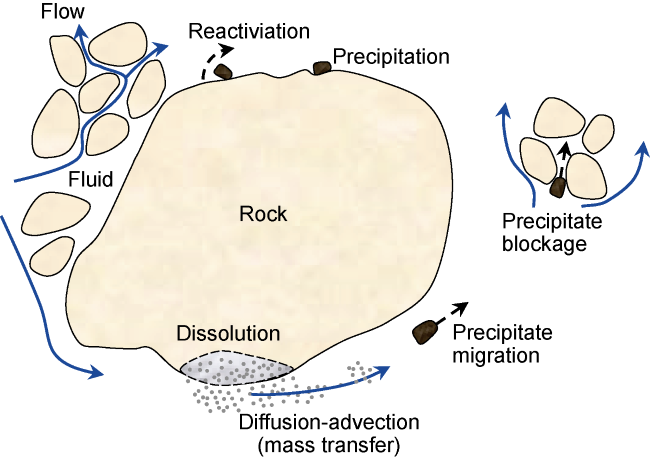
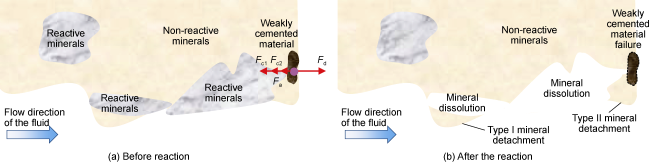
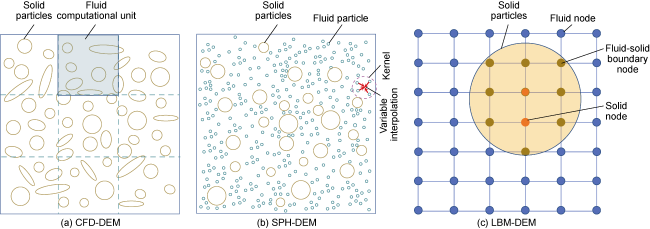
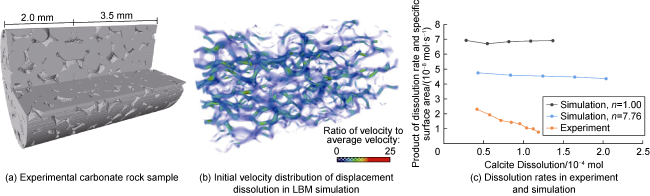
In addition to the above calculation methods, some researchers directly utilize the geochemical simulator PhreeqcRM to obtain reaction rates. Whether directly employing LBM simulation or combining LBM with other CFD discretization methods (e.g. FV, FE, and FD), along with digital core technology and parallel processing techniques, it is feasible to achieve three-dimensional pore-throat simulation of CO
2-water-rock system dissolution reactions at the core scale. This approach enables the quantitative assessment of changes in pore throat, porosity, and permeability induced by dissolution. In static dissolution simulations, Gao et al.
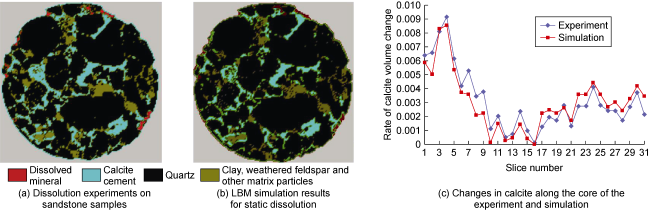
[81] considered the calcite cement in sandstone as reactive, while other minerals were considered non-reactive. They used LBM to simulate the static dissolution process of CO
2-water-rock. The results were compared with dissolution experiments conducted by previous researchers
[82], as shown in
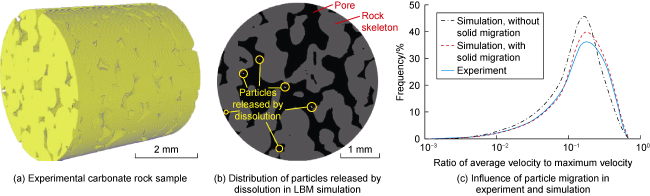
Fig. 7. The model simulated the reaction process between carbonic acid solution and calcite for a duration of 1 440 days, until reaching the equilibrium state of the reaction. Due to the dissolution of calcite, the porosity of the rock core increased from 1.1% to 10.7%. They found that the spatial distribution of calcite in the rock core is crucial. Due to the fact that some calcite is not directly in contact with the acidic fluid, more than one-third of the calcite remained undissolved. In dynamic dissolution simulations, using the pseudo-homogeneous reaction boundary established by Patel et al.
[53], Fazeli et al.
[83] simulated the evolution of fracture dissolution in carbonate rock samples after the injection of CO
2-saturated brine. The results showed that non-reactive minerals present along the flow path can limit the increase in permeability. The commonly used cubic relationship between porosity and permeability in single-mineral porous media fails in multi-mineral porous media. To simulate the CO
2-water-rock reactions at the core scale, An et al.
[84] coupled LBM with the geochemical simulator PhreeqcRM. They evaluated the evolution of porosity and permeability over time under different injection rates, pressures, and temperatures. With increasing flow rates, the dissolution in the rock became more uniform, and they suggested that the permeability increase at high injection rates was larger than that at low injection rates. Xie et al.
[85] employed LBM for fluid flow and component transport and utilized the VOP method to handle the boundary evolution caused by reactions. They simulated the dissolution of calcium carbonate during the CO
2 sequestration process, elucidating that the effect of pressure difference on calcium carbonate dissolution weakened with increasing temperature. They plotted a diagram of the calcium carbonate dissolution pattern based on the relationship between temperature and pressure, suggesting that injecting CO
2 at high temperatures and high injection rates facilitates its dissolution and sequestration. Ju et al.
[86] employed a local reaction boundary format that considers the impact of chemical reaction consumption and reaction reversibility. They simulated the advection-mixing process in the underground saline aquifer for CO
2 sequestration, and analyzed the influence of fluid-rock dissolution reactions and H
2S impurity components on system stability.