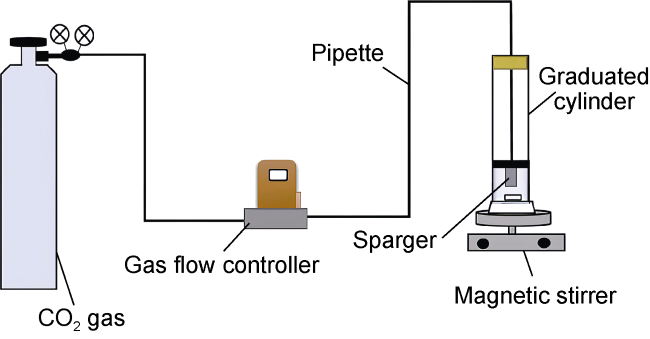
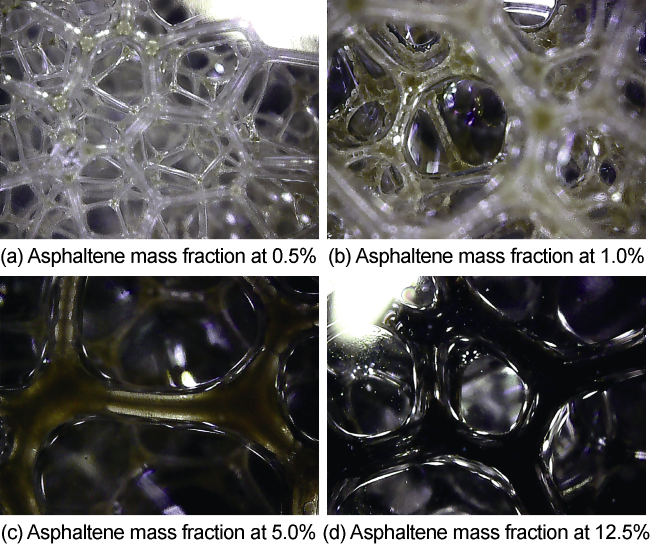
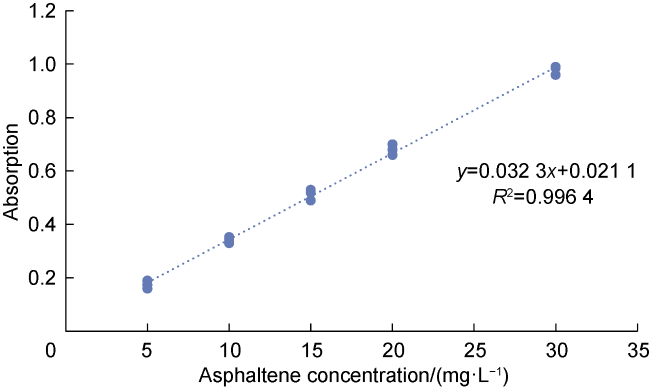
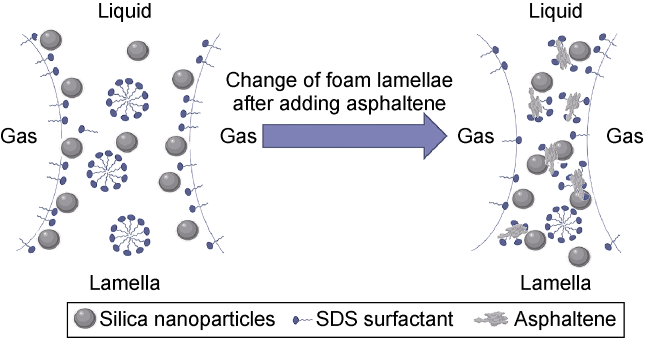
Commonly, nanoparticles are added to foam to improve stability, as nanoparticles can be adsorbed on the surface of the bubbles
[12⇓⇓⇓⇓-17]. In fact, nanoparticles at the gas-liquid interface can minimize the direct contact between the gas and liquid on both sides of lamellae. Moreover, they can reduce the bubble size to increase the foam stability
[18⇓-20]. Many studies have investigated the effects of hydrophilicity/hydrophobicity of nanoparticles on the stability of foams. It has been concluded that compared to hydrophobic nanoparticles, hydrophilic nanoparticles are superior foam stabilizers
[21-22]. Some researchers proved that hydrophobic SiO
2 nanoparticles are highly suitable for producing CO
2 foams because the hydrophobic SiO
2 nanoparticles can be adsorbed at the gas-water interface, producing a more efficient barrier around CO
2 foam bubbles and preventing the destruction of the foam
[23⇓-25]. Bayat et al. surveyed the effect of some nanoparticles, such as TiO
2, Al
2O
3, CuO and SiO
2, on the stability of CO
2-foams, and proved that SiO
2-CO
2 is the most stable foam
[22]. Moreover, the synergistic effect of surfactants and nanoparticles, which is the surface modification of the solid nanoparticles through physiochemical interactions with the surfactants, could increase foam stability and produce stronger foams than the surfactants alone
[26⇓⇓-29]. Kumar et al. investigated the interaction between SiO
2 nanoparticles having negative charges and different surfactants in aqueous solutions
[30]. According to their results, in a nanoparticle-surfactant system with an opposite charge, surfactant adsorption on nanoparticle surfaces can lead to the accumulation of nanoparticles. In contrast, similarly charged nanoparticle and surfactant have no physical interaction
[30]. Interestingly, nanoparticle-stabilized foams are more resistant to harsh reservoir conditions such as high salinity, high temperatures, and the presence of heavy crude oil
[18⇓-20,22,31⇓ -33].