Introduction
1. Geological setting
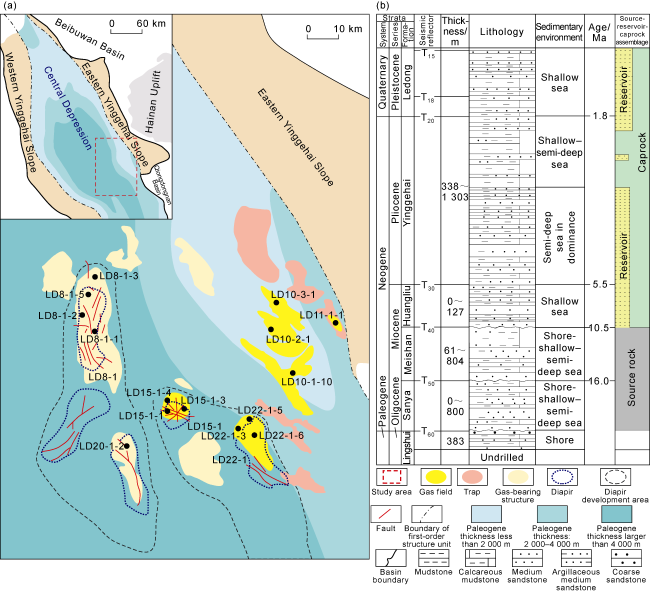
Fig. 1. Structural location (a) and composite stratigraphic column (b) of the Ledong diapir area in the Yinggehai Basin. |
2. Source and geochemical characteristics of helium and its carrier gas
2.1. Source and geochemical characteristics of helium
Table 1. Distribution characteristics of non-hydrocarbon and noble gases in the Ledong diapir area, Yinggehai Basin |
| Area | Forma- tion | Well | Depth/m | N2 content/ % | CO2 content/ % | He content/ % | δ15N2/ ‰ | δ13Cco2/ ‰ | 40Ar/ 36Ar | (3He/ 4He)/ 10−6 | R/Ra | (CO2/ 3He)/ 109 | M/% | L/% | S/% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ledong diapir area | Q1 | LD8-1-2 | 1 194-1 216 | 2.00 | 78.00 | 0.000 7 | −4.0 | −3.3 | 295 | 2.130 | 1.52 | 1.34 | 74.5 | 25.5 | |
| 3.00 | 41.00 | 0.000 7 | −4.0 | −4.5 | 293 | 2.130 | 1.52 | 2.01 | 49.7 | 50.3 | |||||
| LD8-1-3 | 342-352 | 2.27 | 78.90 | 0.000 3 | −5.0 | −2.5 | 292 | 1.390 | 0.99 | 5.44 | 18.4 | 77.4 | 4.3 | ||
| 2.00 | 17.00 | 0.000 3 | −5.7 | −2.5 | 292 | 1.390 | 0.99 | 4.80 | 20.8 | 75.4 | 3.7 | ||||
| LD8-1-5 | 1 115-1 125 | 1.66 | 4.52 | 0.000 4 | −7.0 | −3.3 | 301 | 2.040 | 1.46 | 2.03 | 49.2 | 50.4 | 0.5 | ||
| 2.00 | 7.00 | 0.000 4 | −0.9 | −3.3 | 301 | 2.040 | 1.46 | 2.45 | 40.8 | 56.9 | 2.3 | ||||
| LD8-1-5 | 1 245-1 264 | 3.92 | 59.74 | 0.001 3 | −2.2 | −8.5 | 298 | 0.835 | 0.60 | 3.61 | 27.7 | 50.0 | 22.3 | ||
| LD15-1-1 | 1 417-1 557 | 14.65 | 16.81 | 0.004 5 | −3.3 | −6.9 | 787 | 0.248 | 0.08 | 13.12 | 7.6 | 70.8 | 21.6 | ||
| LD15-1-4 | 1 587-1 605 | 9.80 | 42.04 | 0.004 5 | −3.3 | −6.4 | 787 | 0.105 | 0.08 | 20.74 | 4.8 | 75.0 | 20.2 | ||
| LD20-1-2 | 1 190 | 7.08 | 45.22 | 0.000 5 | −3.0 | −3.4 | 295 | 0.506 | 0.36 | 27.98 | 3.6 | 85.9 | 10.6 | ||
| 1 217-1 220 | 6.24 | 44.70 | 0.000 5 | −3.1 | −3.4 | 295 | 0.506 | 0.36 | 24.67 | 4.0 | 85.5 | 10.5 | |||
| 1 056-1 065 | 4.09 | 52.16 | 0.000 5 | −8.0 | −3.9 | 296 | 0.119 | 0.09 | 68.74 | 1.4 | 85.9 | 12.7 | |||
| LD22-1-5 | 1 595-1 600 | 18.53 | 0.35 | 0.001 9 | −6.0 | −5.3 | 300 | 0.043 | 0.03 | 228.97 | 0.4 | 82.0 | 17.6 | ||
| LD22-1-6 | 587-593 | 18.53 | 0.35 | 0.002 4 | −7.0 | −2.2 | 301 | 0.068 | 0.05 | 113.73 | 0.9 | 92.2 | 7.0 | ||
| N2y- | LD8-1-1 | 1 723-1 737 | 3.78 | 69.33 | 0.001 1 | −5.0 | −3.7 | 302 | 2.190 | 1.56 | 1.36 | 57.9 | 42.1 | ||
| LD8-1-2 | 1 335-1 352 - | 2.00 | 22.00 | 0.001 2 | −6.0 | −4.6 | 341 | 1.470 | 1.05 | 1.36 | 73.5 | 26.5 | |||
| 2.00 | 22.00 | 0.001 2 | −6.0 | −3.7 | 1.470 | 1.03 | 1.73 | 73.5 | 26.5 | ||||||
| LD8-1-5 | 1 245-1 264 | 3.92 | 59.74 | 0.001 3 | −7.0 | −3.3 | 2.040 | 1.46 | 1.92 | 52.0 | 48.0 | ||||
| LD22-1-5 | 1 595-1 600 - | 18.08 | 0.90 | 0.002 1 | −6.0 | −5.3 | 300 | 0.073 | 0.05 | 118.43 | 0.8 | 81.7 | 17.5 | ||
| 16.50 | 0.13 | 0.002 6 | −9.0 | −5.3 | 293 | 0.042 | 0.03 | 148.93 | 0.7 | 81.8 | 17.5 | ||||
| LD22-1-6 | 1 582-1 600 | 16.86 | 0.25 | 0.001 7 | −3.0 | −2.0 | 298 | 0.020 | 0.01 | 486.07 | 0.2 | 93.2 | 6.6 | ||
| 1 468-1 482 | 13.79 | 34.77 | 0.001 7 | −7.0 | −2.2 | 302 | 0.051 | 0.04 | 150.23 | 0.7 | 92.3 | 7.0 | |||
| Ledong Slope area | N1h | LD10-1-10 | 4 000-4 020 | 4.28 | 70.10 | 0.005 4 | −1.8 | 308 | 0.051 | 0.07 | 156.64 | 1.3 | 92.9 | 5.9 | |
| 4 022-4 062 | 4.28 | 70.10 | 0.005 4 | −1.8 | 309 | 0.101 | 0.04 | 79.10 | 0.6 | 93.4 | 6.0 | ||||
| N1m | LD10-2-1 | 4 101-4 170 | 6.85 | 62.17 | 0.005 4 | −0.9 | 775 | 0.025 | 0.02 | 519.66 | 0.2 | 96.7 | 3.2 | ||
| LD10-3-1 | 4 151 | 4.44 | 33.58 | 0.005 4 | −0.8 | 528 | 0.778 | 0.56 | 10.66 | 9.4 | 90.0 | 0.6 |
Note: M, L, and S represent the calculated relative contributions of CO2 components sourced from mantle (mid-ocean ridge basalt, MORB), marine limestone, and organic sediment by MATLAB based on the values of δ13CCO2 and CO2/3He, respectively. |
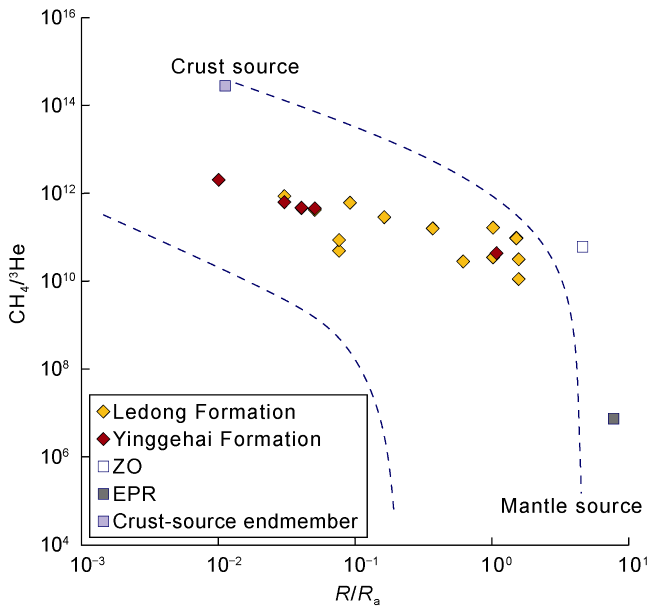
Fig. 2. Correlation of CH4/3He vs. R/Ra of natural gases in the Ledong diapir area, Yinggehai Basin (EPR represents the geothermal fluids of the Pacific Uplift [20]; ZO represents the gas seep in the Zambales Ophiolite Complex, Philippines [21]; the crust-source endmember values refer to Ref. [22]; basemap is modified from Refs. [20,23]). |
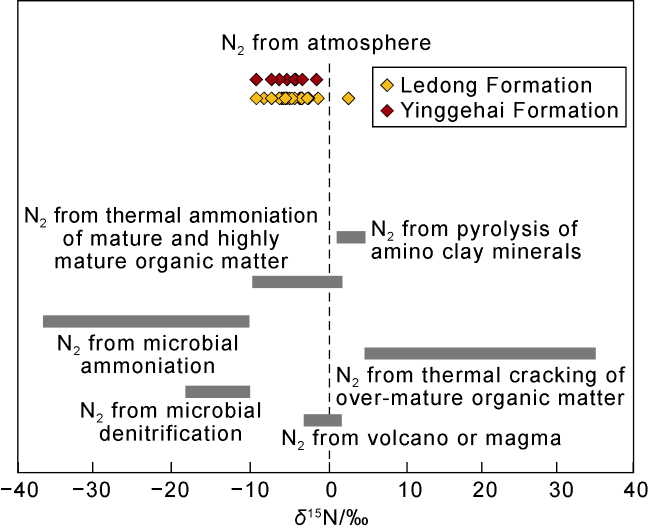
2.2. Carrier gases CO2 and N2
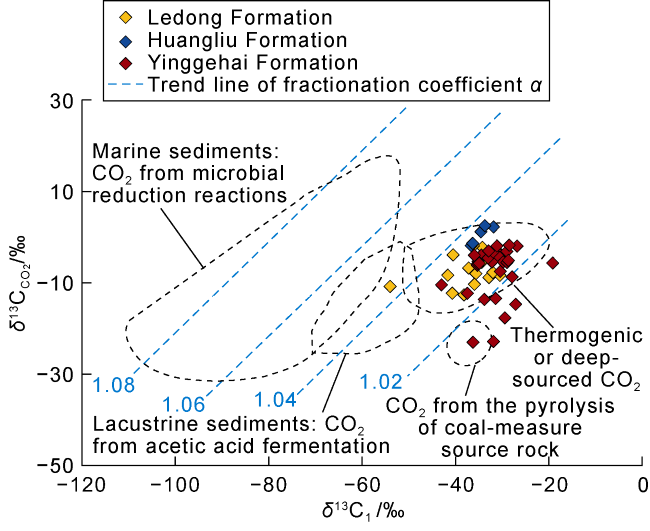
Fig. 3. Correlation of δ13CCO2 vs. δ13C1 values of the natural gas in the Ledong diapir area, Yinggehai Basin (α is the fractionation coefficient of carbon isotope between CO2 and CH4, according to Ref. [29]). |
3. Discussion
3.1. Genetic mechanisms of helium
3.1.1. Thermal driving mechanism of mantle-derived 3He
Table 2. Calculated result of hydrochemical analysis, CO2 concentrations and 3He/Z values in the formation water in the Ledong diapir area, Yinggehai Basin |
| Well | Depth/m | Ion concentration of formation water/(mg•L-1) | pH | CO2(1)/ (10−5 mol·kg−1) | CO2(2)/ (mol·kg−1) | 3He(3)/ (10−12 mol·kg−1) | 3He(4)/ (10−8 cm3·kg−1) | Z(5)/ (kJ·kg−1) | W(6)/ (10−11 cm3·J−1) | XM(7)/ % | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cl− | SO42− | Ca2+ | Mg2+ | K++Na+ | ||||||||||
| LD8-1-1 | 1 723-1 737 | 19 279 | 2 614 | 729 | 1 432 | 10 773 | 6.3 | 3.49 | 0.003 5 | 6.21 | 13.92 | 766.81 | 0.018 | 36.18 |
| LD15-1-3 | 1 447-1 607 | 18 542 | 2 614 | 80 | 611 | 13 611 | 7.7 | 23.90 | 0.466 8 | 2.06 | 4.62 | 313.40 | 0.015 | 29.34 |
| LD15-1-3 | 1 572-1 577 | 4 524 | 679 | 114 | 209 | 3 501 | 7.7 | 16.30 | 0.318 8 | 0.89 | 1.99 | 313.40 | 0.006 | 12.52 |
| LD22-1-3 | 1 486-1 496 | 2 304 | 181 | 160 | 53 | 2 213 | 7.3 | 12.40 | 0.206 7 | 0.99 | 2.22 | 334.38 | 0.007 | 13.10 |
| LD22-1-3 | 579-590 | 19 081 | 2 634 | 457 | 1 224 | 11 139 | 7.0 | 23.00 | 0.798 1 | 0.42 | 0.93 | 238.00 | 0.004 | 7.63 |
Note: (1) CO2 molality in the liquid phase calculated by PHREEQC, according to hydrogeochemical parameters; (2) CO2 molality in the fluid under reservoir conditions calculated by Eqs. (7)-(9); (3) 3He molality in the fluid under reservoir conditions calculated by Eq. (6); (4) 3He concentration converted into the molar volume under STP; (5) Enthalpy value calculated by IAPWS-95 Formulation (according to Refs. [36⇓⇓⇓-40]); (6) The ratio of the initial molar volume of 3He to the corresponding enthalpy under STP, i.e., the initial W; (7) The relative heat contribution from mantle source in the Ledong diapir area, obtained by heat balance equation (Eq. (12)). |
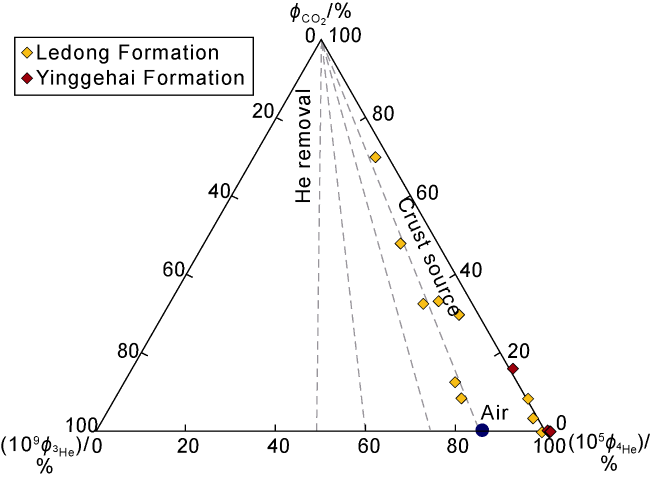
3.1.2. Genetic mechanism of crust-derived 4He
Table 3. Calculated 4He yield rates from wells LD10-3-1 and LD11-1-1 in the Ledong diapir area, Yinggehai Basin |
| Well | Depth/m | Formation | Density/ (g·cm−3) | JTh/10−6 | Ju/10−6 | / (10−13 cm3·a−1·g−1) | / (10−4 cm3·g−1) | /(10−2 cm3·g−1) |
|---|---|---|---|---|---|---|---|---|
| LD10-3-1 | 3 746-4 096 | N1m | 2.55 | 13.85 | 3.06 | 7.66 | 4.10 | 5.84 |
| LD11-1-1 | 3 560-4 778 | N1s-N1m | 2.55 | 14.41 | 3.17 | 7.95 | 4.25 | 9.06 |
3.2. Helium migration mechanism
3.2.1. Primary migration
3.2.2. Secondary migration
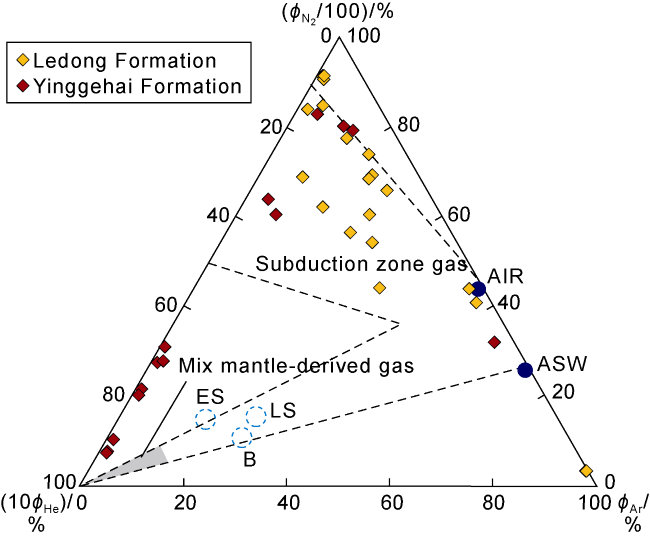
Fig. 6. Ternary diagram of N2, He and Ar in natural gas in the Ledong diapir area, Yinggehai Basin ( |
| Secondary migration | Description |
|---|---|
| Free-gas-phase migration | He of free-gas-phase from primary migration migrates into traps |
| Degassing from helium- bearing groundwater | Migrate with groundwater containing N2: (1) Free-gas-phase from primary migration contacts with groundwater, and then degases from groundwater when contacting other gases (2) Free-gas-phase is dissolved in groundwater, and then absorbs crust-derived gas (N2) and migrates. When the groundwater is oversaturate/water temperature changes/salinity changes/pressure drops/contacts with another gas phase, degassing will occur |
| Gas-liquid separation | Stripping gas from groundwater through migrated CO2 or CH4, that is, CO2 and/or CH4 of free-gas-phase driven by independent buoyancy displace 4He and associated gases from groundwater (helium removal process) |
3.3. Helium accumulation mechanism
3.3.1. Deep thermal-derived helium release and vertical hydrothermal degassing
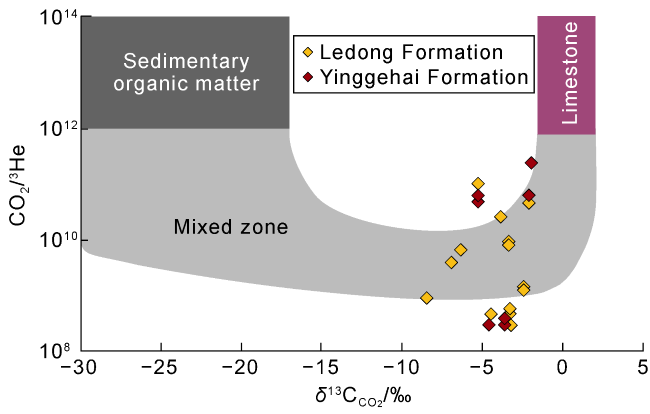
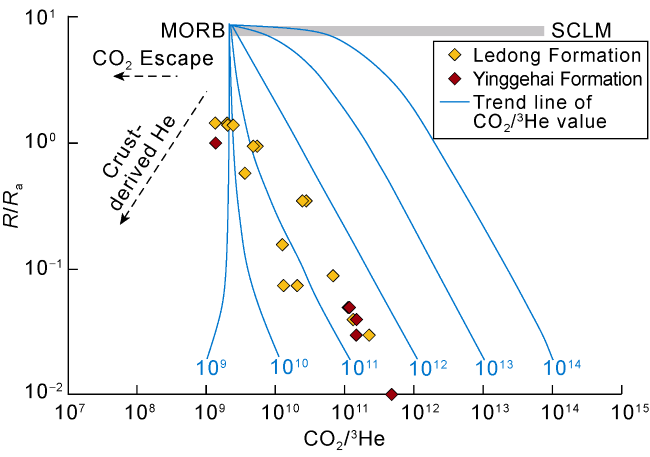
Fig. 7. Correlation of R/Ra vs. CO2/3He values of the natural gas in the Ledong Diapir area, Yinggehai Basin (The base map refers to Ref. [35]; the SCLM endmember indicates the continental lithospheric mantle). |
3.3.2. Lateral migration in shallow layers and accumulation in trap far from faults
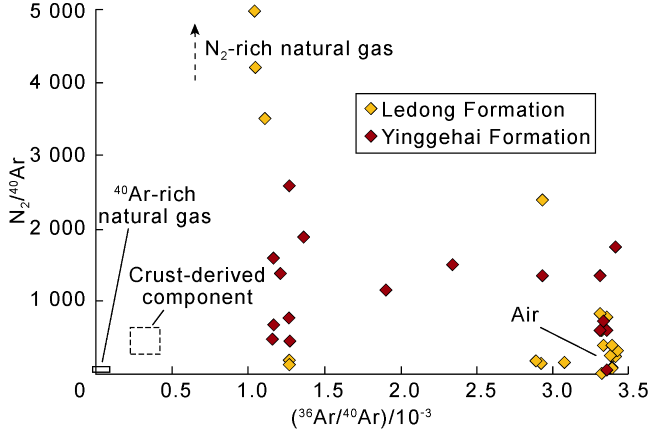
Fig. 9. Correlation of N2/40Ar vs. 36Ar/40Ar in CO2-rich natural gas in the Ledong diapir area, Yinggehai Basin (modified according to Ref. [13]). |