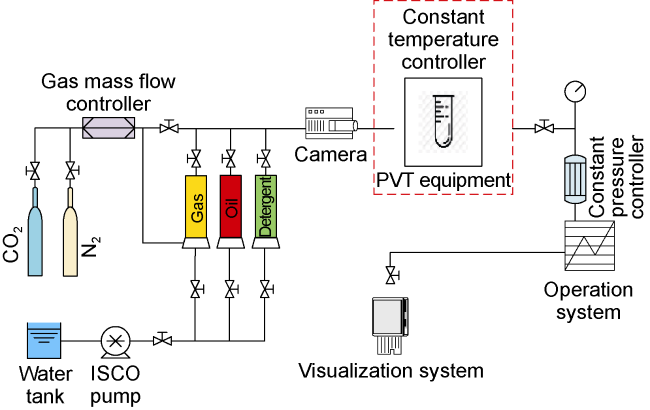
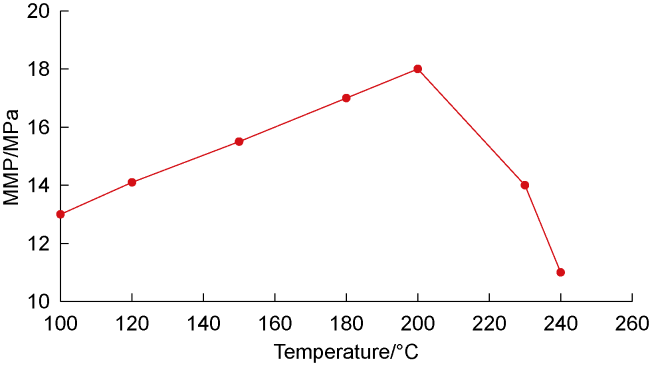
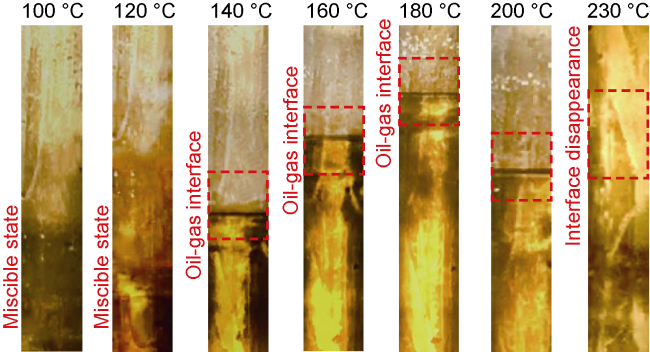
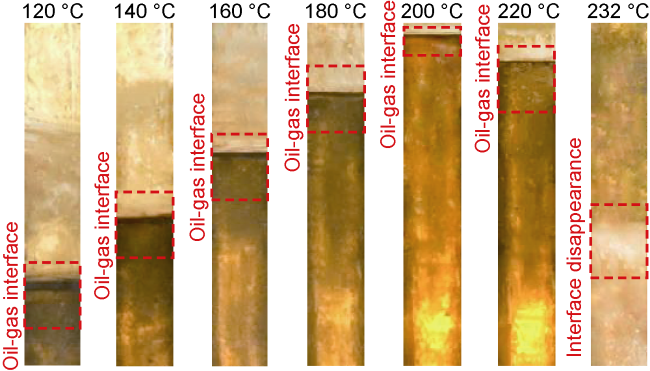
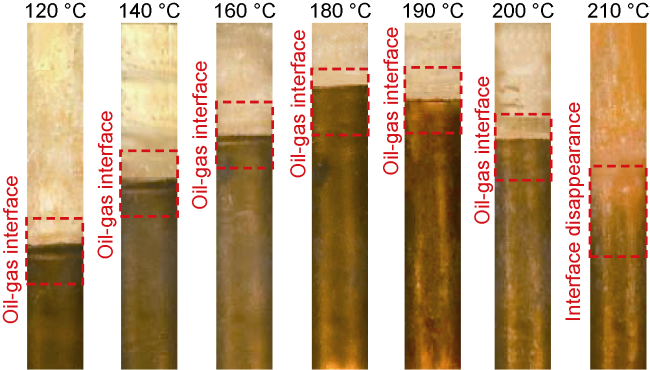
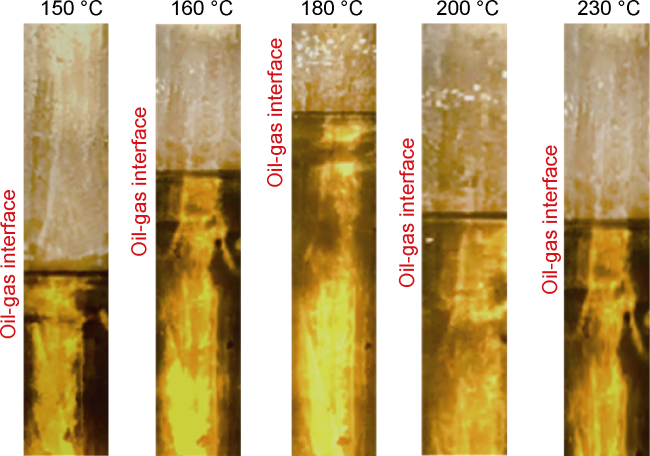
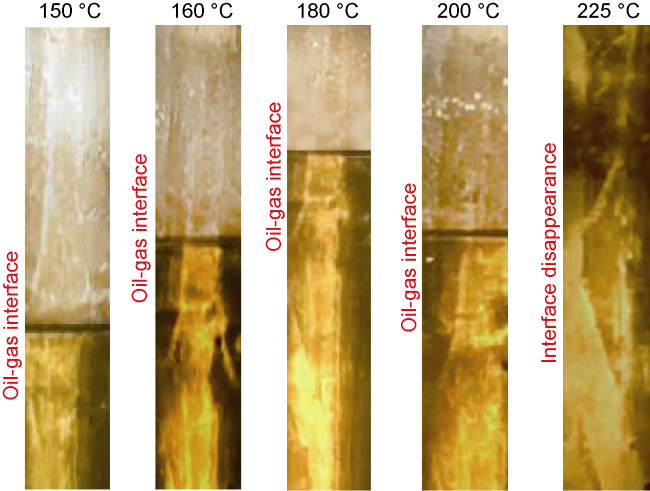
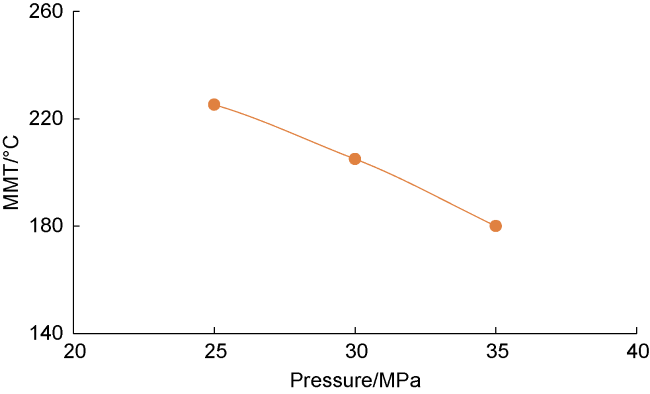
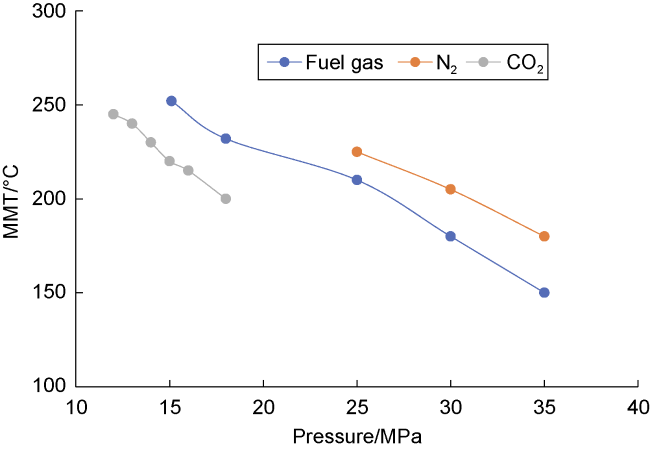
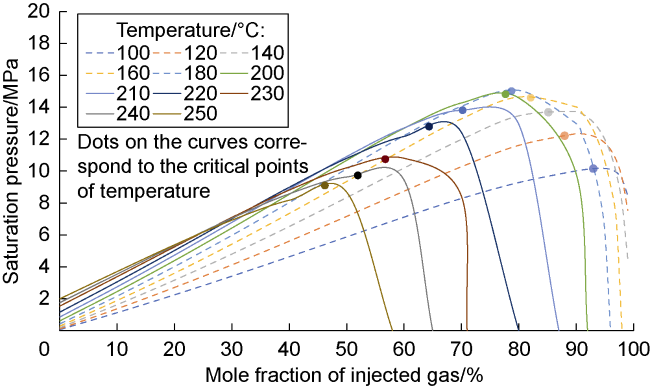
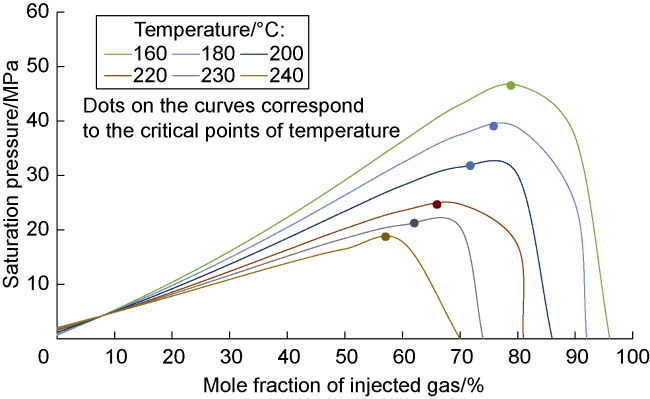
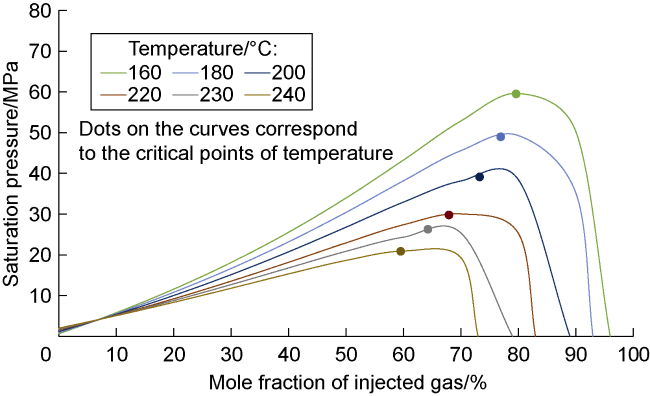
Analysis of the whole experiment process shows that under the pressure of 15 MPa and stepped temperature, the fluids in the PVT cylinder experience a variation from miscibility at low temperature (100 °C) to immiscibility (140-200 °C), and then to miscibility at high temperature (230 °C). In the CO
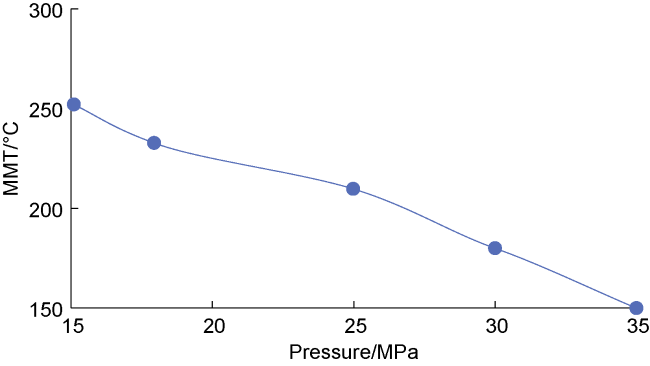
2-condensate oil miscibility experiment, the initial pressure of 15 MPa at 100 °C exceeds the MMP of 13 MPa (
Fig. 2), so the oil-gas system is in a miscible state. Under the same pressure and at low temperature (100-200 °C), CO
2 and oil phases are not miscible when the temperature increasing to 140 °C, which can be defined as the maximum miscible temperature at low temperature range. At 200 °C, distillation and volatilization of light oil components play a leading role, and heating can promote the miscibility. At 230 °C, CO
2 and oil phases reach a miscible state again, which can be defined as the MMT of CO
2 and oil in high temperature range (higher than 200 °C). Once the temperature is higher than the MMT, miscibility can be realized, and temperature rise promotes miscibility. Analysis shows that CO
2 and oil present different miscible states in two temperature ranges. The miscible state below 140 °C is resulted from CO
2-oil interaction under the initial pressure, and miscibility is achieved through dissolution and extraction
[32]. When the temperature is higher than 140 °C, the light hydrocarbon components are distilled and volatilized with the increase of temperature, which is a forced phase transition of light hydrocarbon components, and the miscibility with CO
2 is finally formed. This process is more obvious in the N
2 miscibility experiment.