Introduction
Carbon dioxide flooding is now one of the most promising methods to enhance oil recovery. To date, more than 100 large-scale projects of the CO2 EOR are realized in the world[1]. According to the 2011 United Nations Industrial Development Organization (UNIDO) estimate, the potential of the carbon dioxide-based EOR in the former Soviet Union territory is about 107×108 t; and the projected enhanced oil recovery at giant oil fields is 20%-21%[2].
The carbon dioxide injection technology was implemented at 8 sites in total in the former Union of Soviet Socialist Republics (USSR); and the volume of geological reserves involved amounted to 0.61×108 t or 8% of the total oil production due to the EOR implementation[3]. At the moment, Russian oil companies do not carry out large-scale projects on carbon dioxide flooding; companies are limited to implement single injections using the Huff & Puff technology. However, further deterioration of oil balance reserves and the general industrial rise in Russia will make some day the CO2 EOR widely implemented in this country.
According to Soviet scientists’ reports published earlier, the most likely mode of oil displacement in Russian oil fields would be the immiscible displacement of oil by liquid carbon dioxide[4]. Under immiscible conditions, the “liquid carbon dioxide-oil” mixture decomposes and forms two phases, that is, the heavy oil phase and the light carbon dioxide phase. The phase state of such systems is defined as liquid-liquid equilibrium. In the work performed by J.W. Gardner et al.[5], these phases were termed as the upper liquid and the lower liquid, due to gravitational separation of these liquids in vertically oriented PVT cells. Their terminology will be used throughout this paper. To analyze dynamics of changes in phase properties, initial oil, the single-phase mixture of oil and carbon dioxide as well as pure carbon dioxide will also be named in graphs as the heavy and light phases, respectively.
Orr et al.[6,7,8] concluded through their research results that extraction quantity of hydrocarbons in liquid carbon dioxide is larger than that in gaseous carbon dioxide; in the binary mixture, solubility of individual hydrocarbons decreases as their molecular weight increases and the chains change from alkanes to naphthenes and to aromatic hydrocarbons; in complex mixtures, solubility of alkanes, naphthenes and aromatic hydrocarbons with the same molecular weight differ not much; compared with light components, heavy fractions of oil with high content of aromatic components are extracted less efficiently. Thus, it can be concluded the lower the density of oil, the higher the degree of extraction will be.
Former Soviet scientists also investigated phase-to-phase mass transfer process. Falovsky et al.[9] investigated mass transfer between oil and liquid carbon dioxide in multiple-contact tests. According to the research results, the authors came to the conclusion that the aggregate composition of oil fractions participating in mass transfer remains constant, wherein average molecular weight of extracted oil components increases as the amount of carbon dioxide getting in contact with oil increases; the oil components passing into the light phase are in the same proportions as in the original oil; composition of extractable fractions under other equal conditions almost does not depend on pressure; molar СО2 concentration in heavy phase was equal to the solubility limit of carbon dioxide in the initial oil.
It is known that dissolution of СО2 in oil can decrease oil viscosity and density and increase the oil compressibility and gas content, etc.[10,11]. However, most papers draw attention to the change in oil properties in the area of complete mixing; meanwhile the authors could not discover papers on detailed descriptions of properties of heavy oils that immiscibly interact with carbon dioxide.
In this paper, the authors analyze mass transfer between heavy oil and liquid carbon dioxide. Its novelty is that for the first time quantitatively described were the mass transfer between phases and the change in phase properties for the conditions typical for one of Russian oil fields.
1. Experiment
1.1. Laboratory apparatus
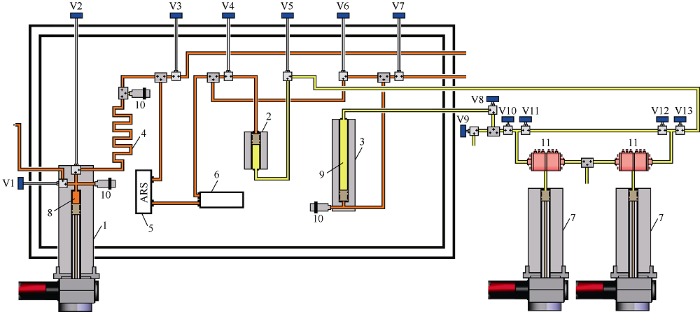
The experiments were carried out via a PVT-unit shown in Fig. 1. The unit is an equipment complex including the following components: high-pressure PVT cells (main cell 1 with volume of 400 mL to measure fluid PVT-ratios, mini-cell of 100 mL and auxiliary cell of 600 mL for fluid circulation in the system); a capillary viscometer (0.3-10 000 mPa•s range of measured viscosities); a digital densitometer (0-3 g/cm3 range of measured densities); an ARS ultrasonic cell designed to record moments of phase transitions by the acoustic resonance method for wax and asphaltenes; a digital video system to record visually the bubble-point pressure of oil; and a personal computer with software to control the unit. All the components of the unit were located in an oven. The oven temperature range was from -10 to 204 °C. The system operating pressure was 103.4 MPa.
Fig. 1.
Schematic diagram of the laboratory apparatus. 1 - main PVT-cell; 2 - auxiliary PVT-cell with a floating piston; 3 - mini-PVT-cell with a floating piston; 4 - capillary viscometer; 5 - ultrasonic system; 6 - digital densitometer; 7 - two-cylinder digital pump to control cells with floating pistons; 8 - reservoir fluid; 9 - hydraulic oil; 10 - pressure and temperature sensors; 11 - electronic valves; V1-V13 - needle valves.
1.2. Oil and gas sampling
The heavy oil sample used in the experiment was taken from wellhead. The reservoir producing the heavy oil has a temperature of 30 °C and pressure of 16 MPa. The sampling interval was 1 582-1 600 m deep, the wellhead pressure and annulus pressure were 1.7 MPa and 0.85 MPa respectively, and the oil recovery mode was cavity pump. The sample has a bubble point pressure of 1.8 MPa, compressibility of 5.22× 10-4 MPa-1, volume factor under formation pressure and bubble point pressure of 1.003 and 1.010 respectively, flash GOR of 2.42 m3/m3, density under formation pressure and bubble point pressure of 961.4 kg/m3 and 954.3 kg/m3 respectively, flash oil density of 961.1 kg/m3, viscosity under formation pressure and bubble point pressure of 795 mPa•s and 513 mPa•s respectively, and flash oil density at 20 °C of 1 574 mPa•s. The composition of the sample is shown in Table 1.
Table 1 Component composition of the oil sample.
| Component | Mol/% | Component | Mol/% | |
|---|---|---|---|---|
| H | 0.03 | i-C4 | 0.73 | |
| H2S | <0.01 | n-C4 | 2.00 | |
| CO2 | 0.55 | i-C5 | 2.12 | |
| N2 | 1.92 | n-C5 | 1.34 | |
| He | 0 | C6 | 4.68 | |
| C1 | 0.52 | C7 | 4.98 | |
| C2 | 0.50 | C8 | 4.71 | |
| C3 | 1.81 | C9+ | 74.11 |
1.3. Measurement procedures
Routine PVT analysis (volume method) and gas chromatography methods were used to test the oil sample. The mixture of the oil and CO2 was prepared under one-time contact (expansion) mode. The constant mass expansion (CME) tests, flash separation and viscosity and density tests under reservoir temperature were done on the heavy oil sample and the mixture.
First, the oil was pumped into the main cell 1, liquid carbon dioxide was dosed into the auxiliary cell 2, the valves V6 and V2 were opened to allow the mixing of oil and CO2 in the main cell. Carbon dioxide concentration in the mixture was monitored by the volumetric method. After pumping a required volume of carbon dioxide into the pump cell, the valve V2 was shut off, and the mixture was stirred with a magnetic stirrer until the cell pressure stabilized, that indicated the equilibrium state was reached. By controlling the quantity of СО2 pumped into the cell, mixtures with different СО2 concentrations of 10%, 26%, 42%, 58% and 75% were prepared. The viscosity and density of the mixtures under formation temperature and pressure dropping from 35 MPa to 1 MPa were tested, and flash separation of these mixtures were done at the pressure of 16 MPa.
When the СО2 concentration was above 10%, the oil and СО2 can’t reach complete miscibility under given conditions and the mixture separated into two liquid phases, that is, light СО2 phase (light phase) and heavy oil phase (heavy phase). The heavy phase volume was measured by transferring the mixture from the main cell to the auxiliary cell. Due to a large difference in viscosity between the phases, a rapid change of viscosity was recorded as the heavy oil phase entered the capillary viscometer. The cell volume corresponding to that change was the heavy phase volume. The light phase volume was calculated as the difference between the mixture volume and the heavy phase volume.
The CME test was done to measure the pressure drop of oil under the same temperature. The procedure measured the bubble-point pressure, relative volume, density, compressibility factor and volume ratio. The bubble-point pressure was measured by a distinctive inflection on the PV isotherm. The relationship between PV of each phase with CO2 concentration was investigated respectively.
Viscosity and density of the phases were measured in the mode of flow between the main cell (1) and mini-cell (3) of the unit. The experiment was done at the same temperature. In the two-phase region, it was not possible to measure the viscosity of the light phase, since its value exceeded the sensitivity limits of the capillary viscometer (less than 0.3 mPa•s).
The procedure of flash evaporation separated the single-phase fluid from the main PVT cell into a separator installed with a gas meter. Based on the experiment results, the gas content, volume factor and properties of the flashed phase were calculated. Component compositions of the separated gas and oil were measured by the gas chromatography method and used to calculate component composition of the heavy oil. All flash separations were carried out at 35 MPa[12,13,14,15]. It was not possible to carry out flash separation of the light phase at carbon dioxide concentration of 26% because of its small volume and high formation volume factor.
2. Analysis of experimental results
2.1. Component composition of phases
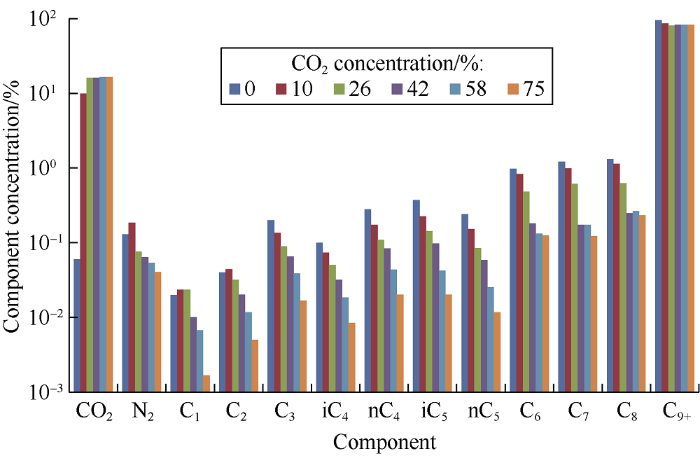
The histogram of heavy phase component compositions for various carbon dioxide concentrations in the mixtures is shown in Fig. 2. The graph shows that the light components - nitrogen and the methane-n-butane series hydrocarbons - behave in roughly the same manner: increase in carbon dioxide concentration leads to diminishing their content in oil, and at concentration of 75% the fall is particularly large. The C6-C8 fractions show a slightly different behavior: their content falls intensely up to carbon dioxide concentration of 42% in the mixture, and after that the fall is decelerated to some extent. The content of С9+ fractions behaves in a special way: it drops sharply to 81.35% at the carbon dioxide concentration of 26%, then increases slightly and almost stabilizes at 82.76%. Solubility of carbon dioxide in oil in the entire range of concentrations is approximately equal to the saturation limit, concentration of 26%, but at concentration of 75% there is a certain increase in the carbon dioxide content (which affects the gas content and volume factor of heavy phases at this concentration).
Fig. 2.
Heavy phase component compositions at various carbon dioxide concentrations in the mixtures.
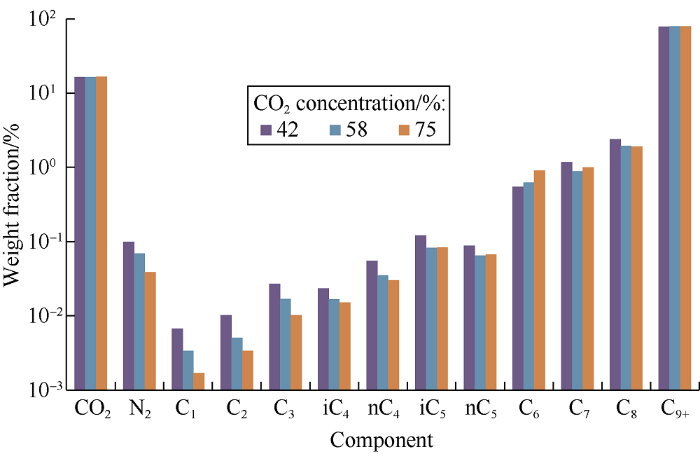
The light phase component composition as a function of carbon dioxide concentration in the mixture is shown in Fig. 3. The figure shows that weight content of nitrogen and С1-С4 hydrocarbons decreases as concentration of carbon dioxide in the mixture increases; С5-С8 hydrocarbons, except for С6, have a minimum content at concentration of 58%; the content of С6 hydrocarbons increases as carbon dioxide concentration in the mixture increases. The С9+ fraction content has a weak maximum at carbon dioxide concentration of 58% in the mixture.
Fig. 3.
Light phase component compositions at various carbon dioxide concentrations in the mixture.
Data on component compositions of the light and heavy phases indicate the following: the molecular weight of the components extracted into liquid carbon dioxide increases as the amount of carbon dioxide that has contacted oil increases. A decrease in the mass content of light components in the light phase is explained by increase in the extractable С9+ fraction.
2.2. Phase and volumetric behavior
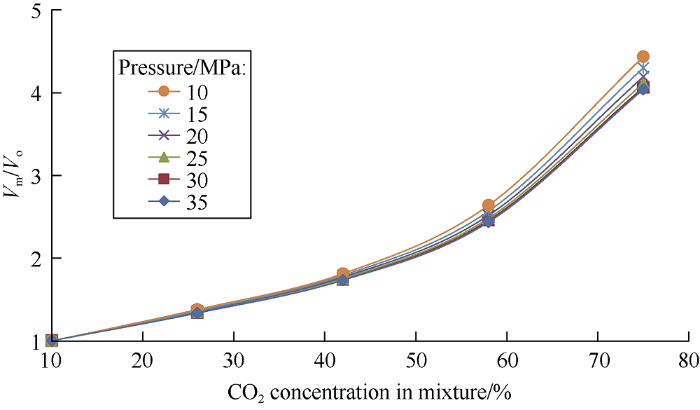
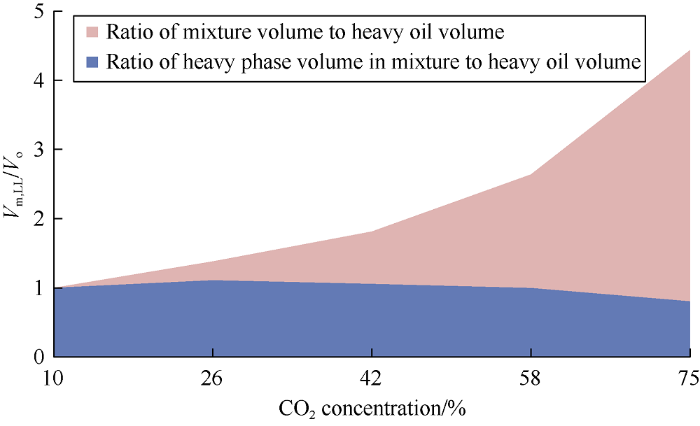
Dependence of mixture volumes to the initial oil volume ratios on carbon dioxide concentrations in the mixture is shown in Fig. 4. It can be seen from Fig. 4 as carbon dioxide concentration in the mixture increases from 10% to 75% and the injection pressure increases from 10 MPa to 35 MPa, the volume of the mixture is 4 times the original oil volume on average, this is mainly caused by the increase of the light phase volume.
Fig. 4.
Dependence of the ratio of mixture volumes (Vm) to the initial oil volume (Vo) on carbon dioxide concentrations in the mixture.
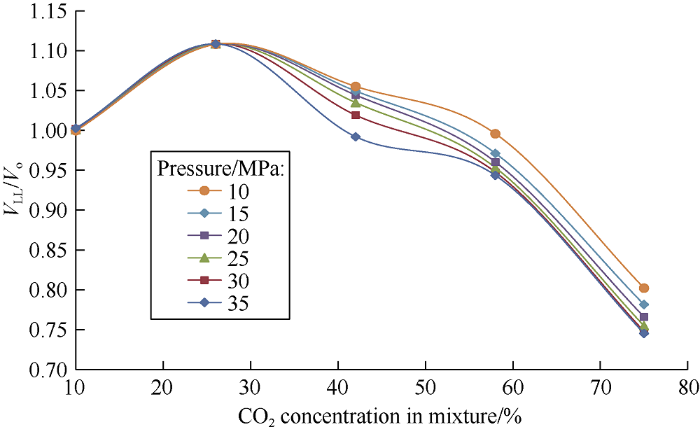
The ratio of heavy phase volumes of mixtures to the initial oil volume against carbon dioxide concentration in the mixture is plotted in Fig. 5. As can be seen from Fig. 5, at carbon dioxide concentration of 10% the oil volume at 15 MPa does not increase (it is 100% of the volume of initial reservoir oil). Apparently, absence of oil swelling at this concentration is due to the large size of the oil molecules[16]. The heavy phase volume increases significantly at carbon dioxide concentration of 10%-26%, this is because the carbon dioxide in the heavy oil has reached the maximum saturation, while light oil components extracted are minimal due to very small amount of liquid carbon dioxide. As the concentration of CO2 increases, the volume of extracted components exceeds the volume of carbon dioxide in the oil, therefore volume of the heavy phase reduces considerably. The relationship between the ratio of mixture volume to the original oil volume at 10 MPa (Fig. 6) shows in the upper pink area the increase of the mixture volume is contributed by the light phase, while in the blue area the increase of mixture volume is attributed to the heavy phase.
Fig. 5.
Dependence of the ratio of heavy phase volume (VLL) to the initial oil volume (Vo) on carbon dioxide concentration in the mixture.
Fig. 6.
Relationship between the ratio of mixture volume to the oil volume and carbon dioxide concentration at 10 MPa.
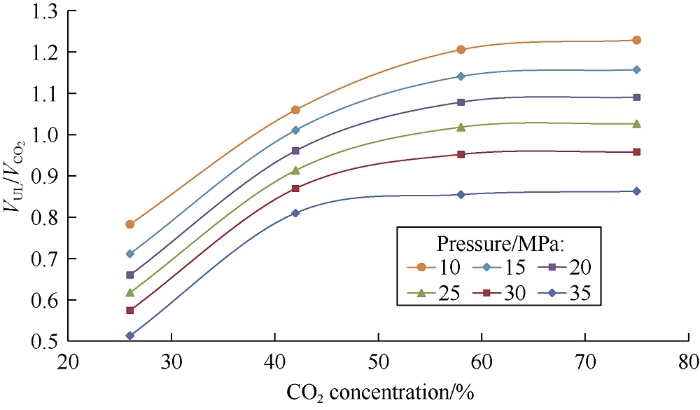
The relative volume of light phase is related to pressure, under different pressures, the relative volume of light phase changes in the same trend with the concentration of carbon dioxide (Fig. 7). The mass transfer between the phases occurs because of the following mechanisms: At the carbon dioxide concentration in the mixture of 26% to 42%, the CO2 in the heavy oil has significant expansion effect but little extraction effect; at the carbon dioxide concentration in the mixture of 42% to 75%, the CO2 expansion in the heavy oil isn’t signi-ficant any more while its extraction strengthens. In the pressure range from 10 to 25 MPa and at carbon dioxide concentration of 42% in the mixture, the swelling of light phase dominates. Under high pressure, compressibility of the light phase is higher than compressibility of pure carbon dioxide, so the relative volume falls below 1 (Figs. 5 and 7).
Fig. 7.
Dependence of the ratio of light phase volume to the carbon dioxide volume on its concentration.
The pressure at the inflection of the PV isotherm is the bubble point pressure (Fig. 8). It can be seen from Fig. 8, as carbon dioxide concentration in the mixture increases, the bubble-point pressure of the heavy phase increases, showing the typical feature of volatile oil with high gas content. In the two-phase region, the bubble-point pressure stabilizes at 7.24 MPa for the light phase and at 7.23 MPa for the heavy phase.
Fig. 8.
PV-isotherms of the mixtures.
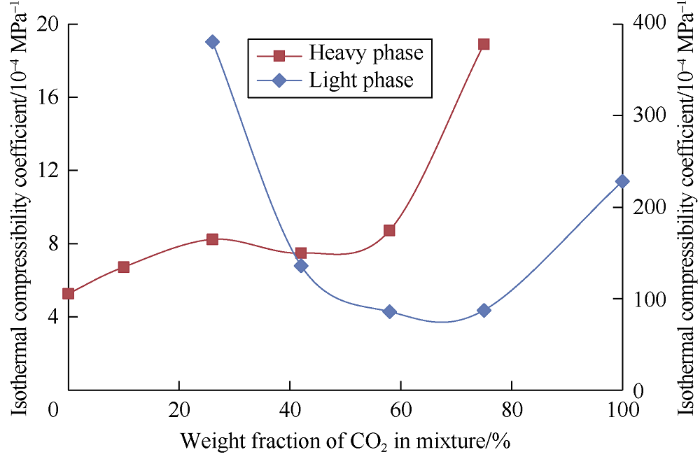
The isothermal compressibility curves of the phases under the pressure range from 7.23 MPa to 35 MPa, CO2 concentration from 10% to 26% and reservoir temperature are shown in Fig. 9. The compressibility curves show under the pressure of 7.23 MPa to 35 MPa, the carbon dioxide concentration from 10% to 26% in the mixture and reservoir temperature, more compressible carbon dioxide dissolves in oil while little oil components is extracted into the light phase. Subsequent decrease in heavy phase compressibility is related to extraction of lightest oil components into the light phase. At CO2 concentration of 75%, compressibility of the heavy phase increases sharply due to a slight increase of carbon dioxide solubility in oil (which is consistent with chromatography data).
Fig. 9.
Compressibility coefficient curve within the pressure range from 7.23 to 35.00 MPa at the reservoir temperature.
Compressibility of the light phase at carbon dioxide concentration of 26% in the mixture is higher than compressibility of pure carbon dioxide and reservoir oil. This is because a lot of light oil components is extracted while little heavy components is extracted into the light phase. Reduction of the light phase compressibility below the compressibility of reservoir oil (at the carbon dioxide concentration from 58% to 75% in the mixture) indicates less compressible heavy oil components are extracted into the light phase as carbon dioxide concentration in the mixture increases.
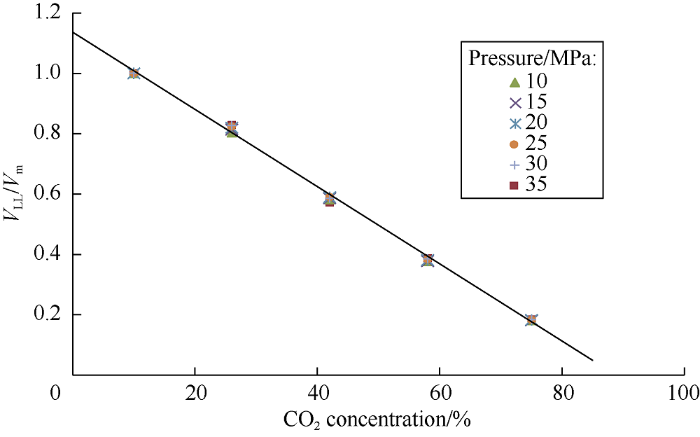
In order to plot mass lines in the phase diagram it is necessary to measure percentage of the heavy phase in the mixture. The ratio of heavy phase volume to the mixture volume under different CO2 concentrations was measured (Fig. 10). The results show that the relative volume of heavy phase is slightly affected by pressure, confirming the Falovsky’s conclusion that composition of extractable fractions hardly depends on pressure when other conditions are the same[17]. Extrapolating the trend line of the heavy phase relative volume at 10 MPa (the line with maximum R2), the carbon dioxide concentration needed to completely dissolve oil in carbon dioxide is 88.4%.
Fig. 10.
The relationship between ratio of heavy phase volume to the mixture volume and carbon dioxide concentration.
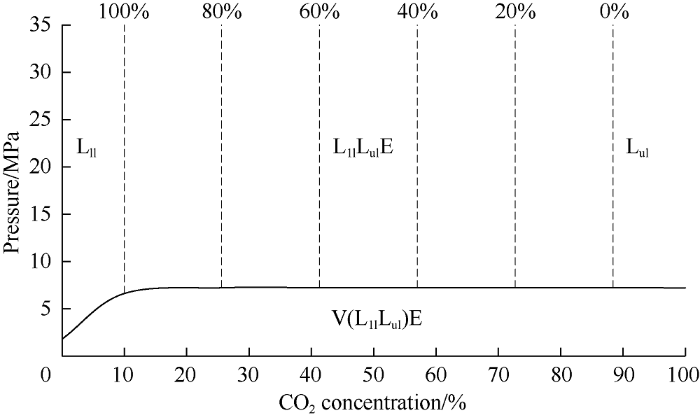
Pressure-composition diagram of heavy oil under low temperature is shown in Fig. 11. In it, the mass line of heavy phase was plotted by interpolating the trend line at 10 MPa. The volume of different phases below the bubble-point pressure were not measured, so behavior of the phase state in the low-pressure range was not analyzed. The phase diagram presented here is very similar to a phase diagram of an oil field in the Saskatchewan province (Canada) compiled by Li et al.[18] (except for the solubility curve of oil in carbon dioxide) .
Fig. 11.
Phase diagram of heavy oil under low temperature.
2.3. Phase behavior under high pressure
Reducing oil viscosity is one of the main effects that enable additional oil to flow into a producing well after injecting carbon dioxide into a reservoir. In the case of incomplete miscible conditions, the ratio of light phase viscosity to heavy phase viscosity would strongly affect the oil displacement efficiency. In the reservoir, the mobility and effective permeability of the light phase are both higher than those of the heavy phase, this will constrain the flow of the displaced fluid (primarily the heavy oil phase). Therefore, to maximize the oil displacement efficiency, the volume of light phase in the mixture needs to be minimized.
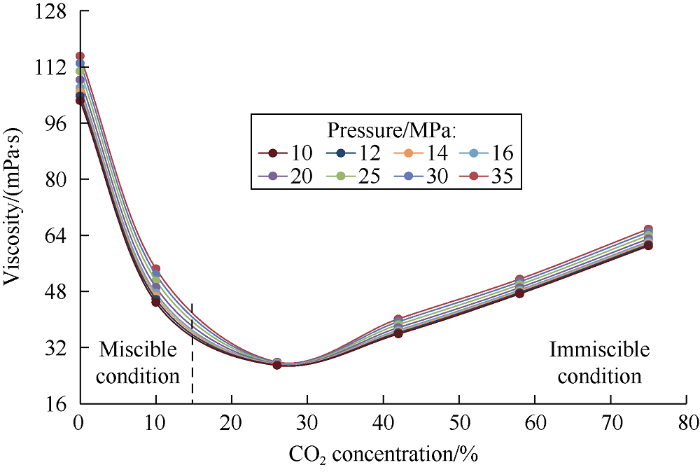
The relationship between viscosity of heavy phase and carbon dioxide concentration in the mixture is shown in Fig. 12. Upon complete dissolution of carbon dioxide in oil (CO2 concentration in the mixture is 10%), the oil viscosity decreases by 91% at the reservoir pressure (16 MPa). In the two-phase region (with CO2 concentration of 26% in the mixture), viscosity of the heavy phase reduces to 96% of the original oil viscosity. As carbon dioxide concentration in the mixture further increases, extraction of oil components into the light phase gets stronger, and the viscosity of heavy phase starts to increase. Viscosity behavior in this region is described by the following expression:
Reliability of the linear approximation values for the equation (1) vary from 0.993 8 at 10 MPa to 0.998 9 at 35 MPa.
Fig. 12.
Dependence of the heavy phase viscosity on carbon dioxide concentration in the mixture.
An essential parameter that describes the fluid is the viscosity variable factor, which is calculated by the following equation:
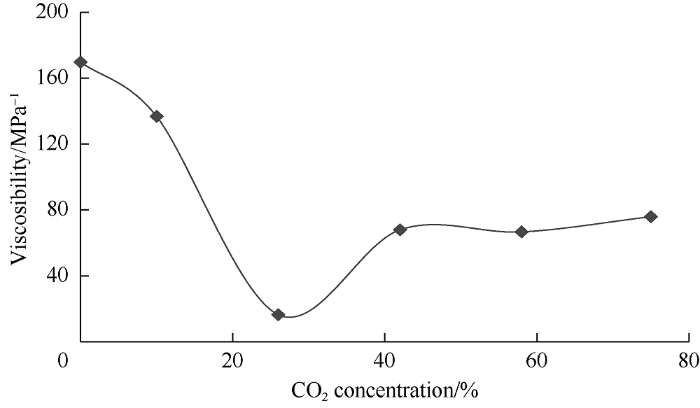
Among other things, viscosity of oil depends on volume ratio of oil components. Light components expand more rapidly than the heavy ones[17], so more light components in oil results in lower viscosity variable factor. Given this, this parameter can also indirectly describe mass transfer between phases. The relationship between viscosity variable factor of heavy phase and carbon dioxide concentration in the mixture in the pressure range from 10 to 35 MPa is shown in Fig. 13. The figure shows that at carbon dioxide concentration of 10% in the mixture, the viscosity variable factor decreases due to the increase in concentration of light component (СО2) in the oil. Maximum decrease of the factor occurs at carbon dioxide concentration of 26% in the mixture, which has been confirmed by the statement given above. As carbon dioxide concentration in the mixture further increases, the viscosity variable factor first increases, then stabilizes, and increases slightly again at CO2 concentration of 75%.
Fig. 13.
Dependence of viscosity variable factor of the heavy phase on carbon dioxide concentrations in the mixture.
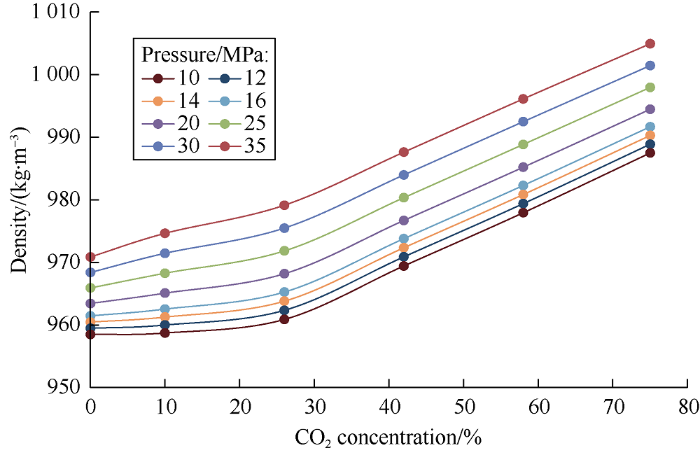
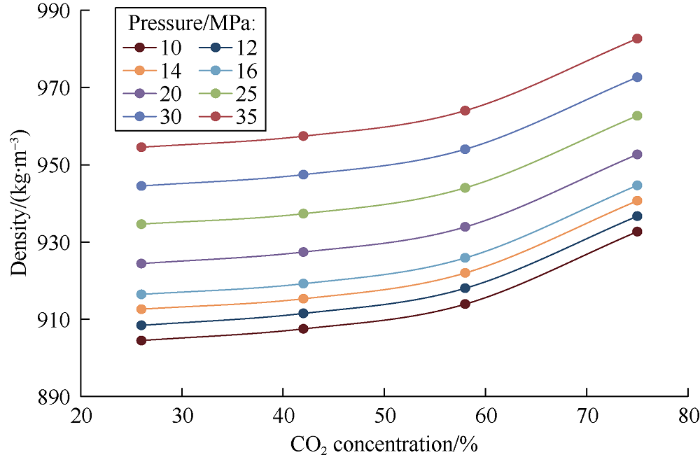
The above graphs illustrate that viscosity is a parameter sensitive to mass transfer between phases. Phase density is an important parameter that affects multiphase flow and, in particular, separation of phases within a reservoir. As carbon dioxide completely dissolves in oil (at СО2 concentration in the mixture of 10% by weight), density of the fluid increases, which confirms the oil doesn’t swell in the carbon dioxide concentrations from 0 to 10% (volume of the heavy phase remains the same). It is interesting to note that as soon as carbon dioxide concentration in the mixture exceeds 26%, the density of heavy phase increases linearly (Fig. 14). With the increase of CO2 concentration in the mixture, the density of the light phase increases in quadratic law (Fig. 15), and with the increase of molecular weight, the heavier oil components gradually dissolve into CO2.
Fig. 14.
Relationship between heavy phase density and carbon dioxide concentration in the mixture.
Fig. 15.
Relationship between light phase density and carbon dioxide concentration in the mixture.
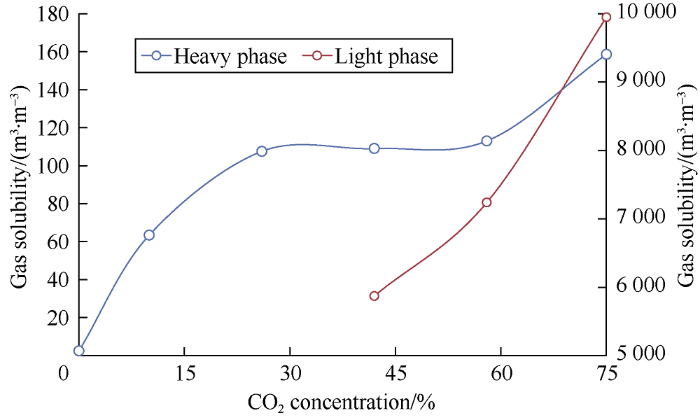
The formation volume factor and gas solubility curves of the heavy and light phases with carbon dioxide concentration in the mixture are presented in Figs. 16 and 17. The formation volume factor and gas solubility curves of the heavy phase and those of light phases are similar in shape. The formation volume factor and gas solubility of the heavy phase increase first (at the CO2 concentration of 0-26%), then stabilize (at the CO2 concentration of 26%-58%), and increase again (at the CO2 concentration of 58%-75%). This is related to an increase in carbon dioxide solubility in oil and may be because the mixture gets close to the saturation limit of carbon dioxide (88.4%) in oil and the mixture changes drastically in properties.
Fig. 16.
Relationship between formation volume factor of the phases and carbon dioxide concentration in the mixture.
Fig. 17.
Relationship between gas solubility of the phases and carbon dioxide concentration.
The light phase behaves a bit differently: its formation volume factor and gas solubility grow constantly with the increase of CO2 concentration in the mixture, which suggests (taking into account low gas solubility in the original oil) that the extraction process cannot compensate for the bulk of carbon dioxide content in the light phase as CO2 concentration increases.
2.4. Properties of flashed phases
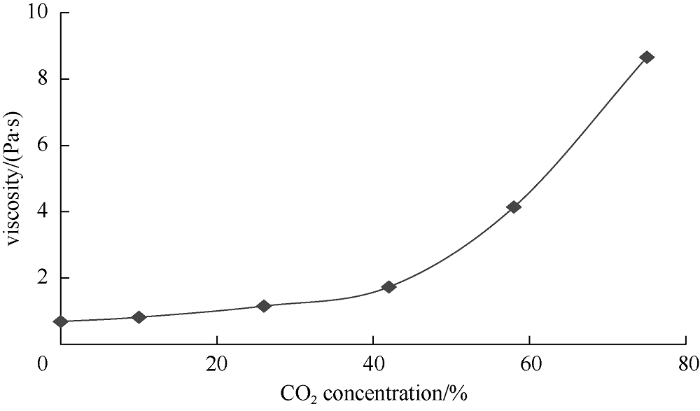
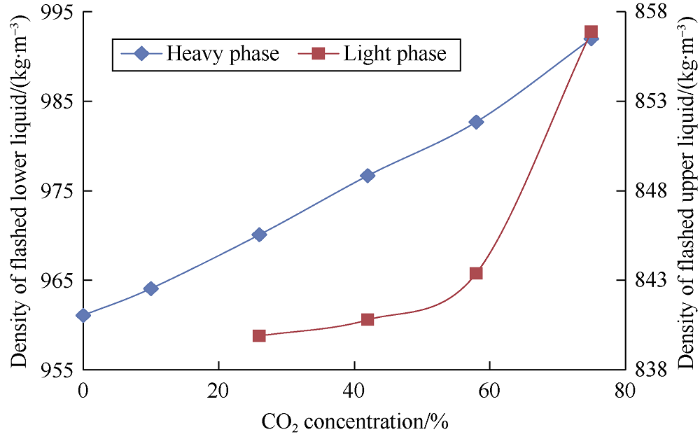
In addition to data on properties of phases under reservoir conditions, data on the flashed fluid is required to implement carbon dioxide flooding, as analysis of this data would help optimize parameters of well operation and oil production systems. The relationships between viscosity of the flashed heavy phase and the flashed phase density and carbon dioxide concentration in the initial mixture are shown in Figs. 18 and 19. The parameters were measured at 20 °C and ambient pressure.
Fig. 18.
Relationship between flashed heavy phase viscosity and carbon dioxide concentration in the mixture.
Fig. 19.
Relationship between flashed phase density and carbon dioxide concentration in the mixture.
It can be seen from the graphs that the viscosity and density of the flashed heavy phase increase continuously as carbon dioxide concentration in the mixture increases. Under complete miscibility of oil and carbon dioxide (CO2 concentration reaching 10% by weight), CO2 escapes from the mixture and entraps light oil components during separation. The viscosity of the flashed heavy phase increases the most at the carbon dioxide concentration of 58% in the mixture, which indicates extraction of oil components by CO2 strengthens. This can cause blockage of surface equipment when producing heavy phase. Density of the flashed heavy phase increases almost linearly and has maximum growth amplitude of 31 kg/m3. In contrast, density of the light phase increases non-linearly, with maximum increase of 7 kg/m3 at carbon dioxide concentration of 75% in the mixture, which indicates that with increase of CO2 volume that has contacted oil, the molecular weight of extracted components increases.
It should be noted that this study does not fully represent multi-contact mass transfer under reservoir conditions. However, it can help us understand fluids’ behavior in the first cycle of the huff & puff process, that is, the mass transfer in carbon dioxide slug injection is not as strong as that in CO2 flooding. A previous study[16] showed that there was a nearly linear relationship between the gas injection rate and the minimum miscibility pressure measured by the slim tube method: the higher the flow velocity of gas in the pore throats filled with oil, the higher the pressure of minimum miscibility would be, hence, the rate of mass transfer would be low at constant pressure. This is consistent with the findings reached from this study. However, the features of oil displacement in the slim tube can’t be regarded same with those in reservoir. The advancement of a gas slug in a reservoir would not be even; first of all, carbon dioxide would get into fractures and the most permeable areas of a reservoir, and the rate of mass transfer would change a little in different injection parts. But still, given that the study results provide the same mechanisms of mass transfer as those of multi-contact mixing, the following conclusions can be drawn:
(1) Bubble-point pressures of the phases are the key parameters that determine oil recovery enhancement of CO2 flooding. When the reservoir pressure drops below the bubble-point pressure, the well turns to dissolved gas drive, leading to significant increase in viscosity of the reservoir fluid and possible blockage of the pore throats by flashed fluid with high viscosity. Given that the reservoir temperature (30 °C) is close to the critical point of pure carbon dioxide (31 °C) and high content of carbon dioxide in the light phase, the light phase can be classified as a near-critical fluid featuring possible avalanche degassing; accordingly, a pressure drop will lead to a sharp increase in the gas-oil ratio and (in presence of water) possible formation of gas hydrate deposits.
(2) As flashed fluid has high viscosity, it is necessary to take proper measures to prevent blockage of surface equipment.
(3) In order to increase profitability and reduce environmental pollution, it is recommended to introduce CO2 trapping technique.
3. Conclusions
The following features in the phase and volumetric behavior of heavy oil interacting with liquid carbon dioxide have been found under the experimental conditions in this study: The oil hardly swells when carbon dioxide completely dissolves in oil (at the CO2 concentration of 10% by weight). When the carbon dioxide concentration in the mixture is over 26%, the heavy phase volume reduces because the volume of oil light components extracted is larger than the volume of CO2 dissolved in the oil, and the viscosity of heavy phase increases exponentially. When carbon dioxide concentration in the mixture is 26%, the reduction of heavy phase viscosity by CO2 is the largest. The density, formation volume factor, gas solubility of light and heavy phases, and viscosity of the flashed heavy phase all increase with the increase of carbon dioxide concentration in the mixture. The production conditions in the heavy oil field suggest the best carbon dioxide concentration in the mixture is 26%, because at this point, the oil swells most and the viscosity of the heavy phase is the lowest. At the carbon dioxide concentration of 10% to 26% in the mixture, the light phase volume is smallest and the oil displacement result is the best.
Nomenclature
B—a factor related to pressure, dimensionless;
${{C}_{\text{C}{{\text{O}}_{2}}}}$—CO2 concentration in the mixture, %;
Cμ—viscosity variable factor, MPa-1;
E—equilibrium phase;
Lll—heavy phase in the lower part of PVT cell;
Lul—light phase in the upper part of PVT cell;
p1, p2—experimental pressures, MPa;
V—gas phase;
μ1—oil viscosity under pressure p1, mPa•s;
μ2—oil viscosity under pressure p2, mPa•s;
μll—viscosity of heavy phase in the lower part of PVT cell, mPa•s.
Reference
A review of CO2-enhanced oil recovery with a simulated sensitivity analysis
DOI:10.3390/en9070481 URL [Cited within: 1]
Global technology roadmap for CCS in industry. Sectorial assessment: CO2 enhanced oil recovery. Melbourne,
Application of EOR methods in Russia and abroad: Experience and prospects
Methodology for the study of oil replacement by carbon dioxide
In:
The effect of phase behavior on CO2-flood displacement efficiency
DOI:10.2118/8367-PA URL [Cited within: 1]
Effect of oil composition on minimum miscibility pressure.
Equilibrium phase compositions of CO2/crude oil mixtures. Part 2: Comparison of continuous multiple-contact and slim-tube displacement tests.
Effect of oil composition on minimum miscibility pressure. Part 1: Solubility of hydrocarbons in dense CO2
DOI:10.2118/14149-PA URL [Cited within: 1]
Aspects of a mass transfer between heavy oil and liquid carbon dioxide
In:
Measurements and correlations of the physical properties of CO2 heavy crude oil mixtures
DOI:10.2118/15080-PA URL [Cited within: 1]
Generalized correlations for predicting solubility, swelling and viscosity behavior of CO2-crude oil systems
DOI:10.2118/917-PA URL [Cited within: 1]
Modeling non-equilibrium phase behavior of hydrocarbon mixtures.
A comparison of various laboratory techniques to measure thermodynamic asphaltene instability.
Minimum miscibility pressure measurement with slim tube apparatus: How unique is the value?.
Asphaltenes, heavy oils, and petroleomics
Determination of multiphase boundaries and swelling factors of solvent(s)-CO2-heavy oil systems at high pressures and elevated temperatures