1. Background
Mercury is a common harmful heavy metal element in natural gas. It is not only toxic but also corrosive. Its existence brings about potential safety hazards to gas field production[1,2]. Among natural gas of various origins, coal derived gas often has a mercury content usually one order magnitude higher than that of oil derived gas[3]. The world's famous high mercury content gas fields are all coal derived gas fields[4-18] (Table 1). For example, the production layer of the Groningen gas field in the Netherlands is Permian Rotliegendian sandstone and the source rock is the Upper Carboniferous coal layers, with an annual gas production of 400×108 m3, natural gas mercury content of 180 μg/m3[4] and annual recovery of liquid mercury about 6 500 kg[5]. In Arun condensate gas field, Indonesia, the production layers are the Middle and Lower Micocene carbonates, the natural gas mainly comes from Baong shale with organic matter of humic kerogen prone to generate gas, and the natural gas mercury content is 180-300 μg/m3[7,8]. In Podravina area of Croatia, the mercury content of natural gas is 200-2500 μg/m3, the source rocks were mainly formed in two periods, the older one formed in the Early Miocene is composed of siltstone and mudstone with type III kerogen, and the newer one is composed of Badenian sediment formed in the Middle Miocene and Pannonian calcareous marl bearing fossils formed in the Late Miocene, optical analysis shows that the main maceral consists of vitrinite and wood debris[9,10]. In the Gulf of Thailand, the natural gas has a mercury content of 100-400 μg/m3[11,12], the strata consist of mainly sandstone and mudstone, and the coal seams are 1.5-3.3 m thick[13]. In the Qasr gas and condensate field of Egypt, the natural gas has a mercury content of 75-175 μg/m3[14], the production layer is the Lower Jurassic Ras Qattara Formation and the middle Jurassic Khatatba Formation, which are all clastic reservoirs, the source rocks are Jurassic Ras Qattara Formation and Khatatba Formation which are mainly composed of shale and sand with coal lines, the types of organic matter are III and II-III[15,16].
Table 1 Statistics on mercury content of natural gas in famous gas fields with high mercury content.
| Nation | Gas field/Location | Natural gas mercury content/(μg·m-3) |
|---|---|---|
| Germany | North of Germany | 1 500-4 350 |
| Netherlands | Groningen | 180 |
| Indonesia | Arun | 180-300 |
| Croatia | Podravina | 200-2 500 |
| Thailand | Gulf of Thailand | 100-400 |
| Egypt | Qasr | 75-175 |
In recent years, with the increasing demand for natural gas in China, great progress has been made in natural gas exploration and development. In all kinds of natural gas, coal derived gas occupies the absolute dominant position, and the harm of mercury is also increasingly apparent. In 2006, a natural gas liquefaction plant in the Fushan Oilfield, Hainan, China, had to stop production and replace the aluminum alloy straight pipe from the main cold box to the gas-liquid separator because of gas leakage. In the process of replacement, liquid mercury was found[2]. The main cold box of Sinopec Yakela gas gathering treatment station also leaked several times in August 2008 and January 2009, resulting in a total of 50 d of shutdown of natural gas processing unit and 2 months of shutdown of the compressor for West-to-East Gas Transmission Project[19]. Therefore, it is not only of great geochemical significance to understand the distribution and origin of mercury in coal derived gas fields in China, but also of great practical significance in today's increasingly stringent requirements for safety and environmental protection.
About the genesis of mercury in natural gas, many scholars have done a lot of work, but because of the lack of comprehensive research, the viewpoints are still divergent. Bailey et al. believed that mercury in San Joaquin Valley crude oil in California was hydrothermal origin, based on the close relationship between hydrothermal mercury deposits and mercury-bearing crude oil in San Joaquin Valley area[20]. Tu Xiuyuan considered that the mercury content in natural gas was closely related to the maturity of oil and gas[21]. Zettlitzer et al. argued that mercury in natural gas in Rotliegand sandstone reservoir in northern Germany originated from volcanic rocks below it[17]. Frankiewicz et al. suggested that mercury in natural gas in the Gulf of Thailand was related to coal and carbonaceous shale near the production layer[22]. Chen Jianfa et al. believed that mercury in natural gas in Liaohe Depression came from the deep part of the earth and had little to do with the type of gas source rocks[23]. Dai Jinxing et al. made statistics on the mercury content of coal derived gas and oil derived gas samples from 12 basins in China and abroad, and found the coal derived gas samples had a mercury content of 0.01-3 000.00 μg/m3 and the arithmetic average is 79.605 μg/m3, the oil derived gas samples had a mercury content of 0.004-142.000 μg/m3 and the arithmetic average is 6.875 μg/m3, the arithmetic average mercury content of coal derived gas was 11.6 times that of oil derived gas, so they reached the conclusion that natural gas mercury content was related to its genetic types[24]. Gou Yanxia et al. argued that mercury in natural gas mainly came from the degassing of mantle-derived magma[25]. Liu Quanyou inspected and analyzed the mercury content in every structural unit of the Tarim Basin and believed that the mercury content in natural gas of the Tarim Basin was mainly related to the genetic type of natural gas, sedimentary environment, tectonic activity and volcanic activity[26].
2. Distribution characteristics of mercury in coal derived gas fields in China
In order to make clear the distribution characteristics and origin of mercury in coal derived gas fields in China, mercury content in more than 500 natural gas wells in eight major gas-bearing basins on land of China was tested. The results show that the highest mercury content is 2 240 μg/m3 and the lowest is less than 0.01 μg/m3. Natural gases of Songliao Basin and Tarim Basin have higher mercury content, gases of the Bohai Bay, Ordos and Junggar basins come second, and gases of the Sichuan, Qaidam and Turpan-Hami basins are lowest (Table 2). This shows that the distribution of mercury in natural gas in China is very uneven, and the mercury content in natural gas varies greatly between different basins and even between different gas fields in the same basin.
Table 2 Statistics on mercury content in natural gas samples from 8 gas-bearing basins in China.
| Basin | Natural gas mercury content/(μg·m-3) | |
|---|---|---|
| Minimum | Maximum | |
| Songliao | Less than 0.01 | 2 240.00 |
| Tarim | Less than 0.01 | 1 500.00 |
| Bohai Bay | 0.20 | 230.00 |
| Ordos | 0.05 | 210.00 |
| Junggar | 1.70 | 110.00 |
| Sichuan | Less than 0.01 | 42.00 |
| Qaidam | Less than 0.01 | 1.42 |
| Tuha | 0.05 | 0.28 |
In order to express easily, the gas fields are divided into H type (with mercury content of greater than 30 μg/m3), M type (with mercury content of 10-30 μg/m3) and L type (with mercury content of less than 10 μg/m3) according to the discussion of mercury content as an identification index of coal type gas and oil type gas made by Han Zhongxi et al.[27,28]. Classification of 8 big gas-bearing basins according to natural gas mercury content and statistics on relevant geochemical parameters (Table 3) show that the distribution of mercury in coal derived gas fields of China has three characteristics: (1) Coal derived gas fields have much higher mercury content in generally than oil derived gas fields, all H type gas fields are coal derived gas fields, such as the Xushen, Changshen and Dehui gas fields in Songliao Basin, the Kera 2, Dina 2 and Yaha gas fields in Tarim Basin, the Nanbu, Suqiao and Banqiao gas fields in Bohai Bay Basin, the Sulige gas field in Ordos Basin, the Mosuowan gas field in Juggar Basin and the Qiongxi gas field in Sichuan Basin. (2) Different coal derived gas fields differ widely in mercury content. Although coal derived gas fields have generally higher mercury content than oil derived gas fields, many coal derived gas fields have low mercury content, belonging to M or L type gas fields, such as the Shuangtuozi and Xiaohelong gas fields in Songliao Basin, the Kekeya, Yingmaili and Ake gas fields in Tarim Basin, the Wangguantun and Rongxingtun gas fields in Bohai Bay Basin, the Yulin, Shenmu, Zizhou, Dongsheng and Daniudi gas fields in Ordos Basin, the Hutubi and Mahe gas fields in Junggar Basin, the Laoguanmiao, Zhebachang Bajiaochang, Hechuan and Longgang gas fields in Sichuan Basin, the Wenxi, Mideng and Hongtai gas fields in Tuha Basin, and the Mabei and Pingtai gas fields in Qaidam Basin. (3) In general, the mercury content in coal derived gas fields increases with the increase of production layer depth. The production layers of all H type gas fields are greater than 2 316 m deep, and those of all M type gas fields are greater than 1 950 m deep. It is worth noting that some coal type gas fields with carbonate reservoirs have very low mercury content although large depth of production layers, for example, Well Wanggu1 in the Wangguantun structure of Bohai Bay Basin, the mercury content of natural gas is less than 0.01 μg/m3 although the depth of production layer is 4 515-4 580 m.
Table 3 Types according to mercury content in natural gas and statistics on related parameters of gas fields in eight major gas-bearing basins in China.
| Basin | Gas field | Production layer depth/m | Formation | Lithology | Gas field type | δ13C2/‰ | Natural gas type |
|---|---|---|---|---|---|---|---|
| Songliao | Xunshen | 3 268-3 705 | K1yc, K1d | Sand, volcanics | H | -34.0 - -31.1 | Coal derived |
| Changshen | 3 498-3 809 | K1yc, K1d | Sand, volcanics | H | -28.8 - -26.3 | Coal derived | |
| Dehui | 2 316-2 328 | K1sh | Volcanics | H | -34.8 - -26.3 | Coal derived | |
| Shuangtuozi | 1 950-2 073 | K1q | Sand | M | -29.1 - -24.3 | Coal derived | |
| Xiaohelong | 613-1 978 | K1q | Sand | L | -28.9 - -24.8 | Coal derived | |
| Lamadian | 600-660 | K2n | Sand | L | -39.8 - -36.6 | Coal derived | |
| Honggang | 540-1 229 | K2n, K2m | Sand | L | -37.4 - -33.3 | Oil derived | |
| Wanjinta | 1 815-1 313 | K1q | Sand | L | Carbon dioxide | ||
| Tarim | Kela2 | 3 499-4 021 | K, E | Sand | H | -19.4 - -17.8 | Coal derived |
| Dina2 | 4 597-5 686 | E | Sand | H | -23.3 - -20.9 | Coal derived | |
| Yaha | 4 947-5 790 | E | Sand | H | -23.9 - -22.6 | Coal derived | |
| Kekeya | 2 983-3 949 | E | Sand | M | -26.6 - -25.7 | Coal derived | |
| Yingmai | 4 452-5 389 | K, E | Sand | M | -20.7 - -24.0 | Coal derived | |
| Ake | 3 250-3 345 | K | Sand | L | -21.9 - -20.2 | Coal derived | |
| Hetianhe | 1 931-2 272 | O, C | Sand, carbonate | L | -34.6 - -30.9 | Oil derived | |
| Tazhong | 3 489-4 973 | O, C | Sand, carbonate | L | -37.8 - -35.1 | Oil derived | |
| Jinan4 | 4 379-4 773 | T | Sand | L | -34.2 | Oil derived | |
| Bohai bay | Nanbu | 4 673-4 689 | Es | Basalt | H | -24.4 | Coal derived |
| Banqiao | 4 917-4 967 | O2f, O2s | Carbonate | H | -26.8 - -26.6 | Coal derived | |
| Suqiao | 4 468-4 856 | O2f, O2s | Carbonate | H | -25.9 | Coal derived | |
| Wangguantun | 4 515-4 580 | O | Carbonate | L | -25.4 | Coal derived | |
| Guxinzhuang | 3 167-3 307 | O2sm | Carbonate | L | Transitional | ||
| Liuquan | 1 500-1 849 | Es | Sand | L | -36.4 - -30.0 | Oil derived | |
| Huanxiling | 2 351-3 042 | Es | Sand | M | -28.1 | Transitional | |
| Rongxingtun | 1 715-1 983 | Es, Ed | Sand | L | -27.7 - -24.7 | Coal derived | |
| Gaosheng | 1 400-1 497 | Es | Sand | L | -34.8 - -32.3 | Oil derived | |
| Ordos | Sulige | 3 288-3 623 | O1m—P2x | Sand, carbonate | H | -24.4 - -23.2 | Coal derived |
| Yulin | 2 677-3 255 | O1m—P2x | Sand, carbonate | M | -26.3 - -23.4 | Coal derived | |
| Shenmu | 2 383-2 845 | P1t—P2x | Sand | L | -27.2 - -22.9 | Coal derived | |
| Zizhou | 1 926-2 713 | P1s | Sand, carbonate | L | -25.7 - -22.7 | Coal derived | |
| Dongsheng | 2 150-2 520 | P2x | Sand | L | -25.6 - -24.5 | Coal derived | |
| Daniudi | 2 350-2 750 | P1s—P2x | Sand | L | -25.3 - -23.8 | Coal derived | |
| Jingbian | 3 150-3 765 | O1m | Carbonate | L | -31.9 - -29.3 | Oil derived | |
| Junggar | Mosuowan | 4 146-4 250 | J1s | Sand | H | -28.0 - -25.5 | Coal derived |
| Hutubi | 3 536-3 614 | E1z | Sand | L | -23.0 - -21.6 | Coal derived | |
| Mahe | 2 410-2 480 | E1z | Sand | L | -25.0 - -24.4 | Coal derived | |
| Sichuan | Qiongxi | 3 682-3 708 | T3x | Sand | H | -22.6 | Coal derived |
| Laoguanmiao | 3 672-3 738 | T3x | Sand | M | -23.7 - -22.8 | Coal derived | |
| Zhebachang | 3 478-4 050 | J1z, T3x | Sand | M | -23.1 - -22.3 | Coal derived | |
| Bajiaochang | 2 544-3 352 | T3x | Sand | M | -27.8 - -26.1 | Coal derived | |
| Hechuan | 2 079-2 191 | T3x | Sand | L | -27.2 - -26.2 | Coal derived | |
| Longgang | 5 955-6 735 | P2ch, T1f | Carbonate | L | -27.0 - -25.3 | Coal derived | |
| Weiyuan | 1 911-3 000 | C, Z | Carbonate | L | -36.2 - -35.7 | Oil derived | |
| Wolonghe | 1 288-4 744 | P2ch—T1j | Carbonate | L | -35.7 - -28.0 | Oil derived | |
| Wubaiti | 4 232-5 045 | P2ch, C2hl | Carbonate | L | -33.6 - -31.0 | Oil derived | |
| Tuha | Wenxi | 2 336-2 358 | J2x, J2q | Sand | L | -26.3 | Coal derived |
| Mideng | 3 062-3 108 | J2x | Sand | L | -26.9 | Coal derived | |
| Hongtai | 2 013-2 067 | J2q, J2s | Sand | L | -25.9 | Coal derived | |
| Qaidam | Mabei | 1 342-1 459 | E31 | Sand | L | -26.4 - -26.2 | Coal derived |
| Pingtai | 1 158-1 161 | E1+2 | Sand | L | -25.1 - -21.4 | Coal derived | |
| Sebei | 709-1 372 | Q | Sand | L | -44.6 - -31.5 | Biogas |
3. Genesis of mercury in coal-type gas fields in China
3.1. Source of mercury in coal derived gas fields
In 2012, the authors discussed the origin of mercury in natural gas and believed that mercury in natural gas mainly came from gas source rocks. With the increase of burial depth of gas source rocks, formation temperature rose continuously, and under the action of thermal force, mercury in gas source rocks migrated with hydrocarbons and accumulated into reservoirs[29]. There are 3 main evidences: (1) Coal derived gas has much higher mercury content than oil derived gas; statistics show the coal derived gas has a mercury content arithmetic average of about 30 μg/m3, oil derived gas about 3 μg/m3, that is to say the mercury content of coal derived gas is one magnitude higher than that of oil derived gas, indicating the genesis of mercury is related to the type of natural gas. (2) The mercury content of natural gas with high carbon dioxide content in Songliao Basin decreases with the increase of carbon dioxide content, indicating that the formation of mercury in natural gas is related to the source of hydrocarbon gas. (3) Coal measures have the material basis for generating natural gas with high mercury content, if calculating according to coal's hydrocarbon productivity and mercury content, the mercury content of natural gas generated by coal can be 6.55- 14077.67 μg/m3, in fact, that is higher than the highest mercury content of natural gas in the world.
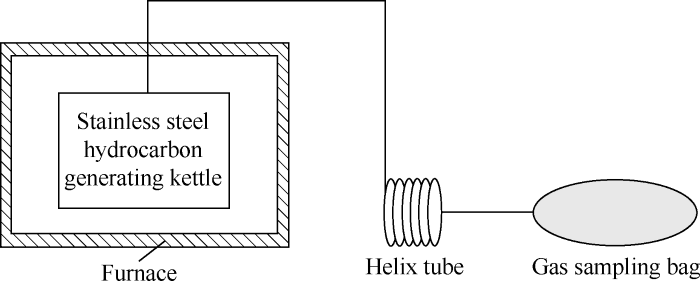
In order to further verify that mercury-containing natural gas can be generated from coal when heated, a modeling experiment of pyrolysis of coal was carried out in this study (Fig. 1). The coal samples used in the experiment were lignite from the Permian in Linfen, Shanxi Province and the Neogene in Zhaotong, Yunnan Province. In the experiment, a certain amount of lignite was added to the stainless steel hydrocarbon generating kettle, and then the kettle was heated in the programmed temperature-controlled heating furnace. The gas released during heating was collected in the gas sampling bag after cooling through the spiral tube. The heating temperature increased from 250 °C at the beginning to 900 °C at last. During the period, gas samples were collected once the temperature rose 50 °C for 1 h at each temperature point. The modeling results show that the mercury content of natural gas generated by lignite from Zhaotong, Yunnan Province was 118 μg/m3 at most, and that of natural gas generated by lignite from Linfen, Shanxi Province was 754 μg/m3 at most. It proves that all coal samples from different areas can generate natural gas with a certain mercury in the process of heating although the mercury in the gases differ in content (Table 4).
Fig. 1.
Fig. 1.
Simulation device of hydrocarbon and mercury generation from coal by heating.
Table 4 Statistics on experiment results of mercury released from lignite by heating.
| Temperature/°C | Mercury content of natural gas/(μg·m-3) | |
|---|---|---|
| Zhaotong, Yunnan | Linfen, Shanxi | |
| 250 | 3.050 | 7.860 |
| 300 | 118.000 | 754.000 |
| 350 | 82.700 | 546.000 |
| 400 | 33.300 | 238.000 |
| 450 | 9.670 | 10.500 |
| 500 | 0.761 | 4.360 |
| 550 | 0.031 | 1.130 |
| 600 | 0.025 | 0.876 |
| 650 | 0.022 | 0.562 |
| 700 | 0.018 | 0.483 |
| 750 | 0.016 | 0.354 |
| 800 | 0.008 | 0.322 |
| 850 | 0.006 | 0.250 |
| 900 | 0.005 | 0.184 |
3.2. Controlling factors of mercury content of coal derived gas
3.2.1. Source rock temperature
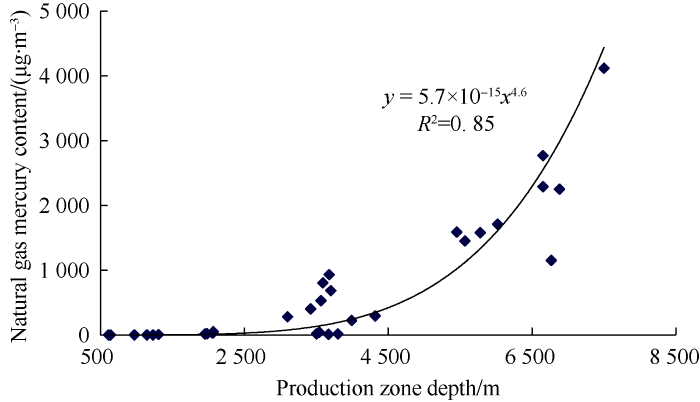
Although the mercury content of coal derived gas is relatively high, the mercury content varies greatly among different coal derived gas fields. The mercury content of coal derived gas is controlled by the depth of production zone. When the depth of production zone is less than 1 700 m, the mercury content of coal derived gas is generally less than 5 μg/m3. With the increase of production zone depth, the mercury content of natural gas increases in a power function relationship (Fig. 2).
Fig. 2.
Fig. 2.
Relationship between mercury content and production zone depth of coal derived gas.
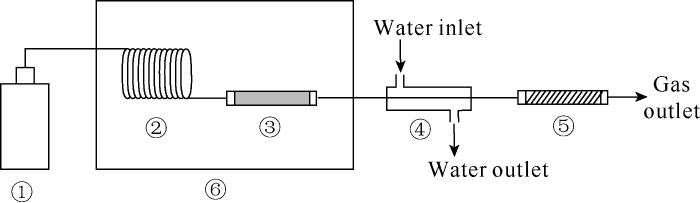
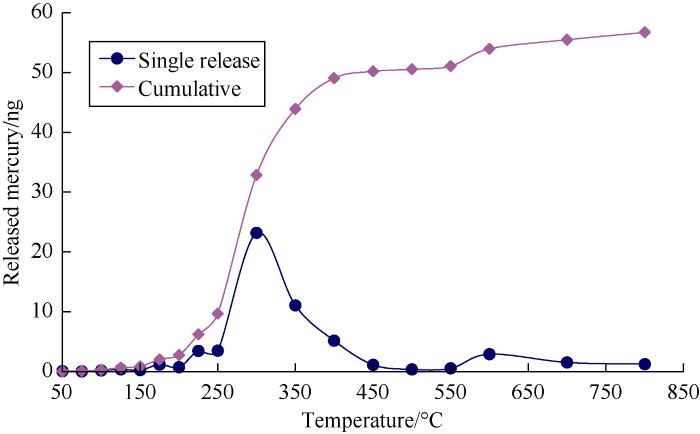
The authors consider that the correlation between mercury content and production depth of coal derived gas in China is essentially related to the process of thermal release of mercury from gas source rocks. In this study, thermal release mercury experiments of coal at different temperatures were conducted (Fig. 3). Firstly, coal was ground and sifted out particles with 0.88-1.70 mm pore size (10-18 mesh); the particles were put into quartz tubes 6 mm in diameter and 18 cm long, the ends of the tubes were sealed with quartz cotton to be coal powder tubes. The coal powder tube was heated for 20 minutes at each temperature point, the mercury vapor released from the coal was swept by nitrogen through the quartz tube loaded with gold wire. The mercury trapping quartz tube was heated to 800 °C, the mercury vapor releasing from the surface of the gold wire was swept into the mercury analyzer by mercury free air. The test results show that the ground coal releases mercury at different temperatures. The higher the temperature, the more mercury was released. The main stage of mercury release is 250-450 °C (Fig. 4).
Fig. 3.
Fig. 3.
Schematic diagram of experimental device for mercury from ground coal by heating.
① Nitrogen cylinder; ② Helix tube; ③ Activated carbon tube; ④ Water-cooled cooling tube; ⑤ Gold wire tube; ⑥ Heating kettle
Fig. 4.
Fig. 4.
Released mercury quantity vs. heating temperature of coal.
3.2.2. Sulfuration environment of the reservoir
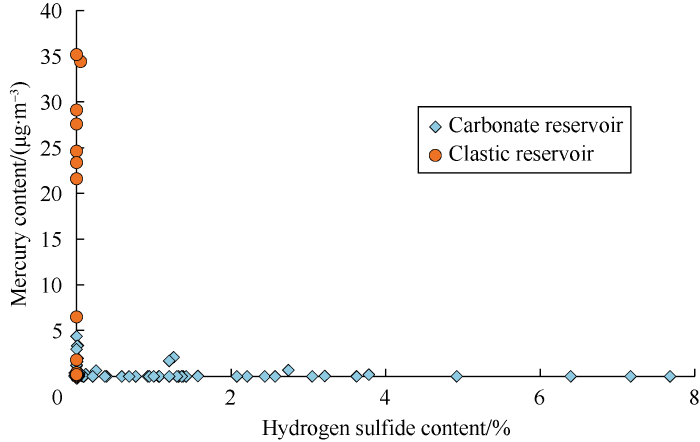
Mercury is a sulfophilic element, cinnabar in nature is formed by the reaction of mercury and sulfur. In carbonate reservoirs, oxidized sulfur, such as gypsum, was converted into reduced sulfur, such as hydrogen sulfide, elemental sulfur,thiophene, mercaptan and thioether, under reducing bacteria and thermochemical sulfate reduction (TSR) environment. When meeting reductive sulfur, mercury in gas is likely to be captured and transformed into mercury sulfide. The stronger the sulfide environment, the lower the mercury content of natural gas. All natural gases containing hydrogen sulfide in Sichuan Basin have a mercury content of no more than 5 μg/m3 (Fig. 5). Although production layer in Well Wanggu-1 of Wangguantun structure, the Bohai Bay Basin has a depth of 4 515-4 580 m, the mercury content of natural gas is less than 0.01 μg/m3 because of the high content of hydrogen sulfide (8.6%).
Fig. 5.
Fig. 5.
The relationship between mercury content and hydrogen sulfide content in natural gas in Sichuan Basin.
Therefore, the mercury content of coal derived gas is mainly controlled by the formation temperature experienced by the source rock and the sulfide environment of the reservoir. The higher the geotemperature the gas source rock experienced, the more the released mercury would be, and the higher the mercury content of natural gas would be, and vice versa. The weaker the sulfide environment of the reservoir, the more likely the mercury in natural gas will be preserved, and the higher the mercury content in natural gas will be. Otherwise, mercury in natural gas will form mercury sulfide with sulfide in reservoir and get lost, resulting in low or no mercury content in natural gas.
3.3. Mercury formation stage in coal derived gas fields
In consideration of the lithospheric material cycle and hydrocarbon formation process, the formation process of mercury in natural gas can be divided into four stages: transport and deposition, shallow burial, deep burial, preservation and destruction.
3.3.1. Transport and deposition stage
During rock weathering or magma eruption, mercury or mercury ions would be transported to lakes, oceans and marshes through the atmosphere, rivers and organisms, and deposit together with organic matter.
The amount of mercury enriched in sedimentary organic matter in this stage is related not only to the types of rock, weathering speed and tectonic activity, but also to the amount and type of organic matter in sediments. In sedimentary organic matter, humus has a strong ability to absorb mercury, as its structure contains a large number of active groups such as hydroxyl (-OH), carboxyl (-COOH), carbonyl (-CO), amino (-NH2) and Mercapto (-SH) which can adsorb by exchange and coordination chelation with mercury[30]. In addition, humus is generally spherical and has a large specific surface area (337-340 m2/g), so its surface adsorption capacity is strong. Therefore, the mercury content in soil depends on the content of humus in soil and sediment. The forest soils with high humus content have a mercury content from 100 to 290 μg/kg, while ordinary soils have a mercury content from 10 to 15 μg/kg[31]. In sediments, sapropel has lower ability to enrich mercury. The average mercury content of coal is no less than 1000 μg/kg, which is more than 12.5 times the Clark value of mercury 80 μg/kg. The mercury content of parent material generating oil derived gas is 150-400 μg/kg, which is much lower than that of coal[32].
3.3.2. Shallow burial stage
With the increase of burial depth, sedimentary organic matter gradually evolves into gas source rocks with gas generating capacity. In the early stage of burial, organic matter still has a strong adsorption capacity to mercury because of its shallow burial depth and low formation temperature. Mercury-bearing gases and hydrothermal fluids rising from the deep crust are further sources of mercury in gas source rocks. These mercury-bearing gases and hydrothermal fluids can be derived from thermal decomposition of non-gas source rocks under the action of formation temperature, or from degassing and dehydration of intrusive magma. Tu Xiuyuan found that the mercury content/organic carbon of the source rocks of He3 Member in Biyang Depression increased with depth[21].
3.3.3. Deep burial stage
With the further increase of burial depth, mercury in gas source rocks will migrate and accumulate together with hydrocarbon gases when the formation temperature reaches a certain level. Han Zhongxi et al. analyzed the phenomenon of mercury absorption and mercury release of ground coal at different temperatures[33], and found ground coal had the ability to absorb mercury when the temperature was lower than 100 °C; when the temperature was higher than 120 °C, ground coal would release mercury rather than absorb mercury; and the equilibrium point of mercury absorption and desorption was about 110 °C[33]. In order to further verify this phenomenon, mercury content in 11 coal samples from Ordos Basin were tested by EPA 7473-2017 EPA Method 7473 Mercury in solids and solutions by thermal decomposition, amalgamation, and atomic absorption spectrophotometry[34]. The results are shown in Table 5. Because of the erosion of the Lower Cretaceous in the Ordos Basin to some extent, the maximum paleotemperature was calculated according to the average erosion thickness of 500 m and the present geothermal gradient, i.e. T=0.029 3h+10.8[35]. The results show that the mercury content in coal increases with burial depth when the maximum paleogeotemperature is less than 106 °C, and decreases when the maximum paleogeotemperature reaches 106 °C.
Table 5 Mercury content and maximum paleogeotemperature of 11 coal samples from the Ordos Basin, NW China.
| Well | Present burial depth/m | Mercury content/(ng·g-1) | Maximum burial depth/m | Paleogeotemperature/°C |
|---|---|---|---|---|
| Shuang8 | 2 251 | 161 | 2 751 | 91 |
| Shen9 | 2 286 | 340 | 2 786 | 92 |
| Yu6 | 2 367 | 363 | 2 867 | 95 |
| Yu40 | 2 551 | 130 | 3 051 | 100 |
| Yu40 | 2 570 | 118 | 3 070 | 101 |
| Yu69 | 2 622 | 520 | 3 122 | 102 |
| Shen12 | 2 636 | 484 | 3 136 | 103 |
| Yu82 | 2 736 | 517 | 3 236 | 106 |
| Yu24 | 2 745 | 204 | 3 245 | 106 |
| Shan245 | 3 177 | 134 | 3 677 | 119 |
| Shan234 | 3 176 | 141 | 3 676 | 119 |
3.3.4. Preservation and destruction stage
When the reservoir temperature is low, the minerals and organic matter in the reservoir may adsorb mercury from natural gas, resulting in the decrease of mercury content in natural gas, even without mercury. When sulphur or sulphide is present in the reservoir, more mercury in natural gas will lose. When the gas reservoir uplifts, leaks or mercury-containing hydrocarbon gas released by deep source rock rise directly to the shallow strata along faults, mercury deposits would be formed under low temperature sulfide environment. The formation of many of the world's mercury deposits might be related to this.
4. Conclusions
The mercury content in coal derived gas in China has three characteristics: the mercury content in coal derived gas is much higher than that in oil derived gas, the mercury content in different coal derived gases vary greatly, and the mercury content in coal derived gas increases with the increase of production layer depth.
The mercury in coal derived gas in China mainly comes from gas source rocks. The modeling experiment of coal hydrocarbon generation reveals that coal can produce natural gas with high mercury content during thermal evolution. The mercury content of coal derived gas is controlled by the temperature of gas source rock and the sulfide environment of the reservoir. The higher the palaeogeotemperature the gas source rocks experienced, the higher the mercury content of natural gas would be; the weaker the sulfide environment of the reservoir, the higher the mercury content of natural gas would be.
In line with the lithospheric material cycle and hydrocarbon formation process, the formation of mercury in coal derived gas can be divided into four stages: transport and deposition, shallow burial, deep burial, preservation and destruction.
Reference
Mercury in petroleum
DOI:10.1016/S0378-3820(99)00068-5 URL [Cited within: 1]
Natural gas hydragyrum (Hg) rejecting technique in Fushan Oilfield of Hainan Province
Composition and its genesis of coal-typed gas
Field detection and implications of mercury in natural gas
Modeling the hydrocarbon generation and migration in the west Netherlands Basin, the Netherlands
Carbonate petrology of Arun limestone, Arun Field, Sumatra, Indonesia
Analytical methods for determining small quantities of mercury in natural gas
Mercury problems in the Arun LNG Plant
Applications of tectonic geomorphology for deciphering active deformation in the Pannonian Basin, Hungary
Tertiary subsurface facies, source rocks and hydrocarbon reservoirs in the SW part of the Pannonian Basin (Northern Croatia and South-Western Hungary)
Removal and treatment of mercury contamination at gas processing facilities
.
Drilling technology applied by American Unocal Company in Siam Bay
Egyptian gas plant employs absorbents for Hg removal
Geochemical characteristics and hydrocarbon generation modeling of the Jurassic source rocks in the Shoushan Basin, north Western Desert, Egypt
DOI:10.1016/j.marpetgeo.2011.07.003 URL [Cited within: 1]
Geochemical characterization of solid bitumen (migrabitumen) in the Jurassic sandstone reservoir of the Tut Field, Shushan Basin, northern Western Desert of Egypt
Determination of elemental, inorganic and organic mercury in north German gas condensates and formation brines
.
Mercury measurements in ambient air near natural gas processing facilities
DOI:10.1007/s002160050087 URL [Cited within: 1]
Improving technique process of mercury removed in Yakela gas condensate treating station
Chemical analysis of brines and crude oil, Cymric field, Kern County, California
The concentration of mercury vapor in natural gas and regolith and its distribution characteristics
The geochemistry and environmental control of mercury and arsenic in gas, condensate, and water produced in the Gulf of Thailand
Origin and geochemical significance of Hg in natural gas from Liaohe Basin
Souce and enrichment condition of mercury in natural gas
Mercury concentration in natural gas and its distribution in the Tarim Basin
DOI:10.1007/s11430-013-4609-2 URL [Cited within: 1]
Discussion of natural gas mercury content as an identification index of coal type gas and oil type gas
New indexes and charts for genesis identification of multiple natural gases
Genesis of mercury in natural gas of Chinese gas fields
Regulating effects of humic acids on the speciation and bioavailability of mercury in soil and its mechanisms
Discussion about direct application of mercury vapor in oil finding: Bureau of Petroleum Survey and Exploration, Ministry of Geology
Analysis of natural gas mercury concentration characteristics from Liaohe Depression
Mercury in solids and solutions by thermal decompostion, amalgamation, and atomic absorption spectrophotometry: EPA 7473—2017
The Ordos basin structure thermal evolution history and its petroleum accumulation significance
DOI:10.1007/s11430-007-6022-1 URL [Cited within: 1]