Introduction
Stimuli-responsive polymers can “feel” stimulus from external environment (temperature, electricity, magnetism, mechanical force, pH, ionic strength, etc.) and change their structures depending on stimulus variation[1]. The structures that could change include short-range structure like chemical composition and molecular configuration, long-range structure like chain conformation and chain size and aggregation structure like texture and crystal. The stimulus-responsiveness of stimulus-responsive polymer has the ability of “recognizing” and “executing”, enabling the polymer to adapt environment variations and function accordingly. It has promising application prospects in fracturing fluid, drilling fluid, leakage plugging, cementing, and enhancement of oil recovery, etc.[2,3,4,5,6,7,8], and is an important mechanism in developing intelligent oil and gas technology[9].
Polymers are the key additives in water-based drilling fluid and are irreplaceable in modifying rheology and controlling fluid loss. However, polymers ubiquitously have poor salt- and temperature-tolerance because of polyelectrolyte effect. In strong brine, the chain conformation of most polymer additives is curled instead of extended, which greatly weakens or even disables polymer’s spatial exclusion effect and multi- point adsorption effect, resulting in ineffectiveness of polymer. Additionally, as brine is a poor solvent for these polymers, the dispersion systems they formed with brine have very bad stability, especially under high temperatures. The introduction of strong hydrophilic, aromatic and heterocyclic groups is an effective way to improve salt and temperature tolerance of polymer[10,11], however, it cannot change the incompatible nature between polymer and salt. In high salinity and high- temperature environment, this method is unsatisfactory, moreover, introduction of large amounts of these groups could bring problems of foaming, bad flexibility and high cost of polymer.
Previously we have developed salt-responsive polymers using three monomers, namely, acrylamide (AM), 2-acrylamido-2-methylpropanesulfonate (AMPS) and 3-acrylamidopropyl trimethylammonium (TAC)[12]. The unique salt-responsiveness enables the salt-responsive polymers to well adapt to salt environment and stand out in salt and temperature tolerance. However, the salt-responsive mechanism of salt-responsive polymers has not been examined in depth. We synthesize a series of AM-AMPS-TAC polymers with the same ionic degree but different charge distributions, and investigate salt-responsive behavior and causes and influence of molecular structure on salt-responsiveness of these polymers through experiments. At last we optimize the AM90-AMPS5-TAC5 polymer and study its application in saturated saltwater drilling fluid.
1. Preparation and characterization of AM-AMPS-TAC polymers
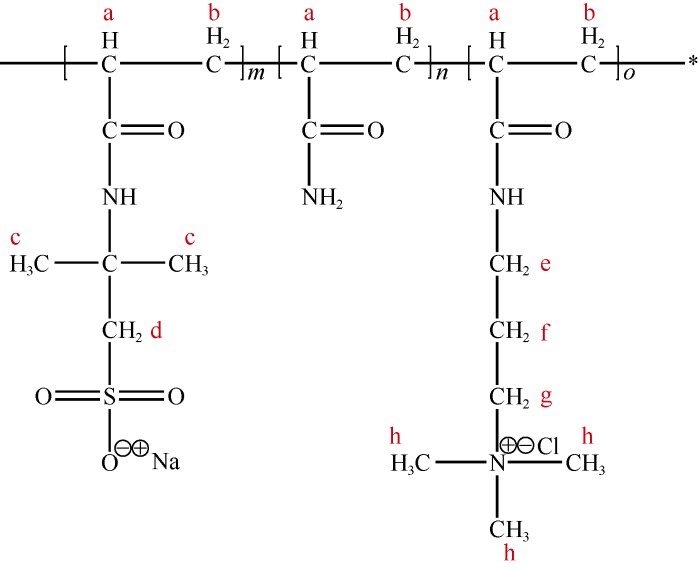
A series of AM-AMPS-TAC polymers were synthesized through aqueous solution polymerization, by controlling molar feed ratios of AM, AMPS and TAC at 90∶5∶5, 90∶6∶4, 90∶7∶3, 90∶8∶2, and 90∶9∶1. The specific synthesis route introduces as follows. (1) 40 g of the three monomers in total (each monomer weight was calculated according to the molar feed ratio) were dissolved in 300 mL of deionized water, and the pH of solution was adjusted to 7.5 with NaOH. (2) 10 g of NaCl as dispersant[8], 0.15 g of HCOONa as chain transfer agent and 50 mg of K2S2O8 as initiator were dissolved in the solution, and high-purity nitrogen was injected to remove oxygen. (3) The solution was sealed and set in water bath at the temperature of 60 °C, and was stirred for 12 h at constant speed for reaction, and viscous gel was obtained. (4) The gel was further purified repeatedly using dialysis bag with molecular weight cutoff of 1000, and then dried and pulverized to get white powder polymer finally. Additionally, following this route, AM-AMPS polymer was synthesized using AM and AMPS at molar feed ratio of 90∶10, with the only difference that dispersant was not added. For convenience, polymer in the following part of this paper is written as AM90-AMPSx- TACy where x and y are molar feed portions of AMPS and TAC respectively (such as AM90-AMPS5-TAC5 and AM90- AMPS10). Fig. 1 shows the general molecular structure of AM-AMPS-TAC polymers, where a-f represents hydrogen atom in different groups respectively.
Fig. 1.
Fig. 1.
General molecular structure of AM-AMPS-TAC polymers.
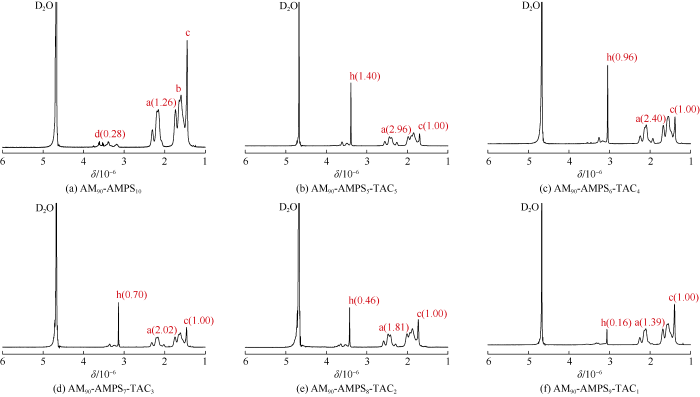
Nuclear magnetic resonance hydrogen spectra (1H-NMR) were used to characterize molecular structure of the polymer using D2O as solvent. Fig. 2a shows 1H-NMR spectrum of AM90-AMPS10, where peak a ((2.04-2.37)×10-6) and peak b ((1.52-1.84)×10-6) represent hydrogen in methyl group and hydrogen in methylene group on the polymer chain backbone respectively (Fig. 1), peak c (1.45×10-6) and peak d ((3.12- 3.79)×10-6) represent hydrogen in methyl group and hydrogen in methylene group on structure unit of AMPS respectively. Fig. 2b, 2c, 2d, 2e and 2f shows 1H-NMR spectra of AM90- AMPS5-TAC5, AM90-AMPS6-TAC4, AM90-AMPS7-TAC3, AM90- AMPS8-TAC2 and AM90-AMPS9-TAC1 respectively. All of them show peak h which represents hydrogen in methyl group on structure unit of TAC, and show overlapped and undistinguishable peaks representing hydrogen in methylene groups on structure units of AMPS and TAC (d, e, f and g). These results indicate that all the polymerizations are successful, and all the polymers are purified fully because the peak representing hydrogen on alkene group ((4.50-6.50)×10-6) doesn’t appear.
Fig. 2.
Fig. 2.
1H-NMR spectra of AM-AMPS-TAC polymers (data in bracket is peak area).
2. Salt-responsiveness of AM-AMPS-TAC polymers
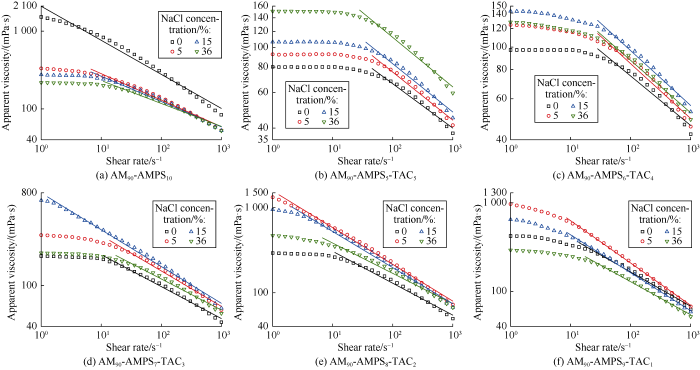
The salt-responsive polymer solution increases in viscosity, while solution of conventional polymer decreases in viscosity with the increase of salt stimulus strength[7]. To study salt- responsiveness of the AM-AMPS-TAC polymers, the relationships of viscosity with shear rate of their solutions (at mass fraction of 2%) were measured with HAKKE rheometer, and the influences of NaCl addition (0, 5%, 15% and 36% (saturated)) on rheology of the polymer solutions were investigated as well. The measurements were carried out in stepwise mode, that is, 30 steps were taken to change the shear rate from 1 000 s-1 to 1 s-1 and each step lasted for 0.5 min. Apparent viscosity at a corresponding shear rate was obtained by calculating the integral average.
As AM90-AMPS10 is an anionic polymer, its solution decreases in viscosity with the increase of salt content because of polyelectrolyte effect (Fig. 3a). In contrast, all the AM- AMPS-TAC polymers show salt-responsiveness with different degrees. AM90-AMPS5-TAC5 solution has the strongest salt- responsiveness and increases in viscosity with the increase of salt (Fig. 3b). Solutions of the other AM-AMPS-TAC polymers increase and then decrease in viscosity with the increase of salt. For example, at the shear rate of 1000 s-1, AM90- AMPS6-TAC4 solutions with 0, 5%, 15% and 36% of salt have a viscosity of 42.3, 45.6, 52.8 and 48.9 mPa·s respectively (Fig. 3c). The turning point corresponds to salt concentration between 15% and 36%, and viscosity variations at other shear rates also show this change pattern. Comparing Fig. 3c-3f, it can be seen that with the increase of molar feed ratios of AMPS and TAC, the salt concentration at which the viscosity variation of polymer solution turns from increase to decrease tends to go down.
Fig. 3.
Fig. 3.
Influence of NaCl concentration on rheology of AM-AMPS-TAC polymer solutions.
Besides viscosity, shear-thinning performance of the polymer solutions varies significantly as well under salt stimulus. As shown in Fig. 3, all the solutions increase first and then keep constant in viscosity with the decrease of shear rate, and the Newtonian platform is caused by insensitivity of the polymer to weak shear. The dividing point between Newtonian section and non-Newtonian section can be determined by extending two parts of the rheology curve. The slope of non-Newtonian section is obtained by linear fitting. The influence of salt addition on shear-thinning performance is investigated by comparing the slope, namely, smaller slope indicates stronger shear-thinning. It is can be seen that with the increase of salt, the shear-thinning of AM90-AMPS10 solution becomes weaker, whereas the shear-thinning of AM90-AMPS5- TAC5 solution becomes stronger, and the shear-thinning of other polymer solutions turns stronger first but then weaker.
These variations are consistent with variations of viscosity.
3. Chain conformation variations of AM-AMPS- TAC polymers
Conformation is the statistical spatial arrangement morphology of polymer chain derived from internal rotation of single bond, which is one of the major factors affecting macroscopic properties of polymer[13]. Generally, the more extended the chain conformation of the polymer solution, the bigger the excluded volume and the higher the viscosity of the polymer solution will be. Conversely, the more curled the polymer chain, the lower the viscosity of the polymer solution will be. From the results of rheology measurements, it is found that the AM-AMPS-TAC polymers show different chain conformation variations under salt stimulus. With the increase of salt addition, chain conformation of AM90-AMPS10 curls constantly, whereas chain conformation of AM90-AMPS5- TAC5 extending continuously, and chain conformation of the other polymers extends first but then curls.
The variation regularity of chain conformation was verified by testing turbidity of polymer solution (20 g/L). As shown in Table 1, the turbidity of AM90-AMPS10 solution without salt is basically zero, indicating a true solution, which means that AM90-AMPS10 chain extends fully in water. After salt addition, because of polyelectrolyte effect, the polymer chains begin to curl, leading to decreasing solubility and higher turbidity of the polymer. Without salt, the turbidity of AM90-AMPS5-TAC5 solution reaches 152.0 NTU, and the turbidity becomes lower with the increase of salt addition, which proves its chain conformation extends with the increasing salinity. The other polymer solutions increase first and then decrease in turbidity with the increase of salt addition, which proves that they extend first but then curl in chain conformation.
Table 1 Turbidity variations of polymer solutions with NaCl concentration.
| Polymer | Turbidity of solution/NTU | |||
|---|---|---|---|---|
| CNaCl= 0 | CNaCl= 1.5 mol/L | CNaCl= 3.0 mol/L | CNaCl= 4.5 mol/L | |
| AM90-AMPS10 | 0.2 | 0.8 | 1.3 | 1.9 |
| AM90-AMPS5-TAC5 | 152.0 | 6.8 | 1.1 | 0.6 |
| AM90-AMPS6-TAC4 | 107.7 | 5.5 | 1.1 | 1.8 |
| AM90-AMPS7-TAC3 | 44.4 | 5.1 | 7.7 | 8.2 |
| AM90-AMPS8-TAC2 | 9.8 | 2.1 | 3.8 | 5.2 |
| AM90-AMPS9-TAC1 | 1.7 | 1.7 | 3.9 | 4.1 |
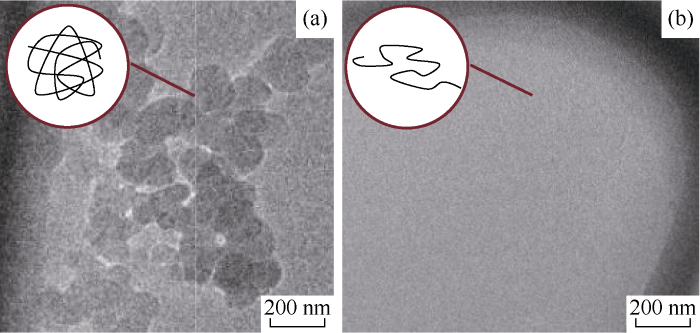
The microcosmic morphology of AM90-AMPS5-TAC5 fresh water solution (CNaCl=0) and brine solution (CNaCl=4.5 mol/L) in Table 1 was analyzed with cryo-transmission electron microscope (cryo-TEM) to further confirm variations of chain conformation (Fig. 4 shows enlarged pictures of polymer chains). It can be seen that the polymer is not molecularly- dissolved as it presents curled conformation in the fresh water solution, but disperses in microspheres instead, the solution becomes turbid as the polymer microspheres refract light (Fig. 4a). In the high concentration brine, the polymer chains extend fully, forming true solution. Under this circumstance, microspheres disappear and the solution becomes clear (Fig. 4b).
Fig. 4.
Fig. 4.
Microcosmic morphology in AM90-AMPS5-TAC5 fresh water solution (a) and brine solution (b).
4. The salt-responsiveness mechanisms
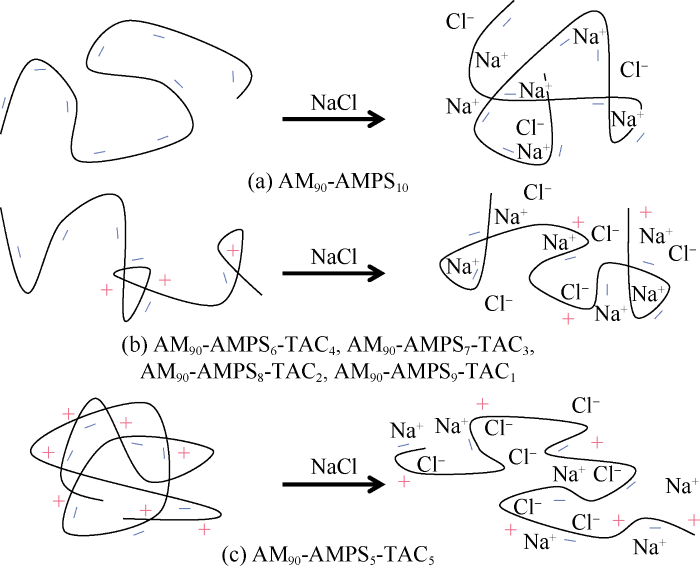
Based on above discussion, the chain conformation of AM- AMPS-TAC polymer turns from curled to extended under salt stimulus. This phenomenon often appears in “poly-innersalt” which has intramolecular ionic bond (N+ and SO3-) in the structure unit[7], resulting in chain curling in fresh water. But the chains can extend in high concentration brine as the ionic bond is weakened by charge screening effect of lots of small ions (Na+ and Cl- ionized from NaCl).
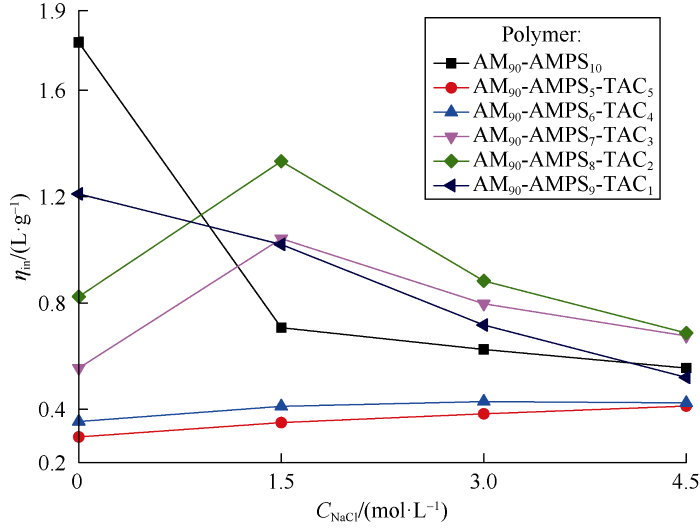
As AM-AMPS-TAC polymer is polyampholyte which carries randomly-distributed cationic structure units (TAC) and anionic structure units (AMPS) after ionization in water[8], ionic bond could either exist in the same chain or between different chains. To study the causes of salt-responsiveness, the variation of intrinsic viscosity ηin with NaCl concentration was examined. The intrinsic viscosity was measured according to the following procedures: NaCl solutions at polymer mass fractions of 1.0, 1.5 and 2.0 g/L and constant volume were prepared, apparent viscosity η of the polymer solution and solvent viscosity η0 at 1 000 s-1 were tested with Fluidicam microfluid visible rheometer[14], relative viscosity ηr and specific viscosity ηsp of the polymer solution were calculated[13], and the final ηin was obtained by calculating arithmetic average of two intrinsic viscosities from linear fitting based on Equation (1) and Equation (2).
It can be seen from Fig. 5 that the intrinsic viscosity of AM-AMPS-TAC polymer changes in similar pattern as viscosity shown in Fig. 3 with the increase of NaCl concentration. The intrinsic viscosity indicates the contribution of a single polymer chain to the solution viscosity, which excludes the influence of interaction between different polymer chains on viscosity, so the salt-responsive behavior of AM-AMPS-TAC polymer derives from chain conformation extension caused by the weakening of intramolecular ionic bond. The higher the salt concentration, the higher the weakening degree of ionic bonds and the more extended the polymer chains will be.
Fig. 5.
Fig. 5.
Variations of polymer intrinsic viscosity with NaCl concentration.
The experiment results above indicate that the salt-responsiveness of AM-AMPS-TAC polymer is influenced by its molecular structure as well, and the difference of molecular structure is caused by the difference in molar feed ratio of anionic monomer AMPS and cationic monomer TAC. However, the feed ratio of monomer isn't always consistent with its actual structure unit ratio in the polymer. Thus, copolymer constitution was analyzed. Characteristic peak c (-CH3, 6H) representing structure unit of AMPS and characteristic peak h (-CH3, 9H) representing structure unit of TAC were integrated, and further the actual ratios of AMPS structure unit and TAC structure unit in the polymer were calculated by comparing the peak areas according to Equation (3). The ratio of AMPS structure unit in AM90-AMPS10 was calculated based on areas of peak a and peak d.
When the feed ratios of AMPS and TAC are 5∶5, 6∶4, 7∶3, 8∶2, 9∶1 and 10∶0, the actual ratios of corresponding structure units in the corresponding polymers are 5.6∶5.2, 6.8∶4.5, 8.2∶3.8, 9.2∶2.8, 12.0∶1.3 and 11.1∶0 respectively. It can be seen that the feed ratio of AMPS to TAC is very close to the actual ratio of AMPS structure unit to TAC structure unit in the corresponding polymer, which proves that the polymer structure difference is from the difference of ratios of structure units with positive and negative charges. This result also indicates that AMPS and TAC have close reaction activity in this polymerization, which probably because of the same acrylamido structure of them.
Under screening effect of small ions, both the polymer intramolecular electrostatic repulsion and electrostatic attraction (ionic bond) which cause chain curling and chain extension respectively are weakened. AM90-AMPS10 only has electrostatic repulsion, so its chain conformation keeps curling with the increase of salt stimulus strength, with no salt-responsiveness. AMPS structure units and TAC structure units in AM90- AMPS5-TAC5 are basically the same in number, resulting in neutrality of its polymer chain and domination of electrostatic attraction. Under this circumstance, the polymer has the strongest salt-responsiveness, and thus its chain conformation keeps extending with the increase of salt stimulus strength. The other polymers, with both repulsion and attraction, have some degrees of salt-responsiveness. They show composite variations in chain conformation with the increase of salt stimulus strength. The chain conformation variations of polymers with different molecular structures under salt stimulus are illustrated schematically in Fig. 6.
Fig. 6.
Fig. 6.
Schematics of chain conformation variations of polymers with different molecular structures under salt stimulus.
5. Application
The bentonite in water-based drilling fluid would dehydrate in high salinity environment because its diffusion double layer is compressed, resulting in severe flocculation, sharp increase of gel strength and uncontrollable fluid loss. Salt-tolerant polymer additives have colloid-protecting effect and can adsorb onto bentonite surface to maintain the dispersion of bentonite in high salinity environment[16]. To evaluate salt- and temperature-tolerance of AM-AMPS-TAC polymers in drilling fluid, the polymers (mass fraction of 2%) were added into bentonite base mud (mass fraction of 4%) saturated with NaCl, the base mud was further hot-rolled at 150 ℃. The variations of apparent viscosity, Ф6 and Ф3 of the base mud with hot-rolling time were tested by Fann viscosimeter. Bigger readings of Ф6 and Ф3 indicate stronger structure of bentonite, and more combination of bentonite in “face-to-face” pattern and higher flocculation degree.
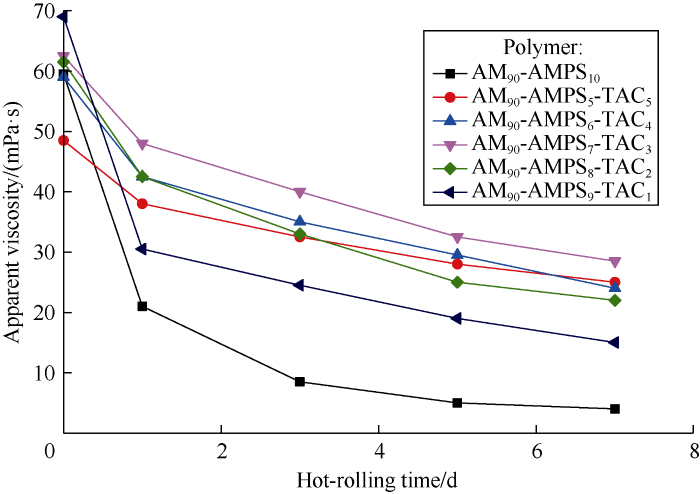
It can be seen from Fig. 7 that the apparent viscosity of the base mud with anioinc AM90-AMPS10 decreased 64.4% after 1 d of hot-rolling, and decreased almost to zero after 5 d of hot- rolling, which indicates that AM90-AMPS10 is highly-degradable and thermally-unstable. By comparison, the apparent viscosity of base mud with AM-AMPS-TAC polymer reduced much slower after hot-rolling, indicating that AM-AMPS- TAC polymer can maintain stability for longer time. It can be seen from Table 2, Ф6 and Ф3 readings of the base mud with AM90-AMPS10 were zero after 7 d of hot-rolling, indicating no flocculation, because debris of degraded AM90-AMPS10 contain no cationic groups prone to flocculation and sulfo-group contained in AMPS has strong dispersing ability. In contrast, Ф6 and Ф3 readings of the base mud with AM90-AMPS5-TAC5 were basically unchanged after 7 d into hot-rolling; Ф6 and Ф3 readings of the base mud with AM90-AMPS9-TAC1 after 7 d into hot-rolling increased by 40% and 50% respectively; Ф6 and Ф3 readings of the base mud with other AM-AMPS-TAC polymers doubled after 3 d into hot-rolling in general, indicating severe flocculation. In summary, AM90-AMPS5-TAC5 has the best salt- and temperature-tolerance since it makes the bentonite base mud saturated with NaCl stable in viscosity and maintains good dispersion. In addition, the API fluid loss of the base mud with AM90-AMPS5-TAC5 decreased from 119.0 mL to 7.8 mL before hot-rolling, and was 11.2, 14.3, 17.8 and 26.6 mL after 1, 3, 5 and 7 d of hot-rolling respectively, indicating that AM90-AMPS5-TAC5 has good performance in controlling fluid loss.
Fig. 7.
Fig. 7.
Variations of apparent viscosity of NaCl saturated bentonite base mud with different polymers with hot-rolling time.
Table 2 Ф6 and Ф3 readings of NaCl saturated bentonite base mud with different polymers with hot-rolling time.
| Hot-rolling time/d | Ф6/Ф3 | |||||
|---|---|---|---|---|---|---|
| AM90-AMPS10 | AM90-AMPS5-TAC5 | AM90-AMPS6-TAC4 | AM90-AMPS7-TAC3 | AM90-AMPS8-TAC2 | AM90-AMPS9-TAC1 | |
| 0 | 5/4 | 4/3 | 4/3 | 4/3 | 5/4 | 5/4 |
| 1 | 2/1 | 4/3 | 6/5 | 9/8 | 7/5 | 5/4 |
| 3 | 1/0 | 4/3 | 9/8 | 10/9 | 9/8 | 6/5 |
| 5 | 1/0 | 5/3 | 10/9 | 12/10 | 10/10 | 7/5 |
| 7 | 0/0 | 5/4 | 11/9 | 13/11 | 11/10 | 7/6 |
Three kinds of saturated saltwater drilling fluids were prepared with AM90-AMPS5-TAC5 and PAC-Lv which is the most commonly-used fluid loss control agent for water-based drilling fluid. The formulas are: 1#, 2% bentonite+0.5% NaOH+1.5% AM90-AMPS5-TAC5+3% white asphalt+saturated NaCl+barite; 2#, 2% bentonite+0.5% NaOH+1.5% PAC- Lv+3% white asphalt+saturated NaCl+barite; 3#, 2% bentonite+0.5% NaOH+1.2% AM90-AMPS5-TAC5+0.3% PAC- Lv+3% white asphalt+saturated NaCl+barite. Table 3 shows the basic properties of the drilling fluids. The saturated saltwater drilling fluid with only AM90-AMPS5-TAC5 and white asphalt (1#) has high viscosity. The saturated saltwater drilling fluid prepared with PAC-Lv (2#) has hardly any gel strength, namely, has poor performance at ambient temperatures, and isn’t usable, which make it meaningless to conduct hot-rolling. The saturated saltwater drilling fluid prepared with AM90- AMPS5-TAC5 and PAC-Lv (3#) has controllable viscosity, and good basic properties, indicating that AM90-AMPS5-TAC5 and PAC-Lv have good synergistic effect and that AM90-AMPS5- TAC5 can enhance the function of PAC-Lv to some extent. The final saturated saltwater drilling fluid prepared by adding barite into the 3# drilling fluid successively has a density range of 1.80-2.20 g/cm3, proper rheological parameters and good temperature-tolerance. This drilling fluid has little change of all parameters after hot-rolling and low fluid loss (lower than 15 mL).
Table 3 Basic properties of saturated saltwater drilling fluids.
| Drilling fluid formula | Test condition | Density/ (g·cm-3) | Apparent visco- sity/(mPa·s) | Plastic visco- sity/(mPa·s) | Yield point/Pa | (Initial gel strength/ final gel strength)/Pa | High-temperature high- pressure fluid loss/mL |
|---|---|---|---|---|---|---|---|
| 1# | Before hot-rolling | 1.80 | 88.5 | 70 | 18.91 | 4.0/6.5 | — |
| After hot-rolling | 84.5 | 67 | 17.88 | 4.0/6.0 | 7.8 | ||
| 2# | Before hot-rolling | 1.80 | 17.5 | 17 | 0.51 | 0.5/0.5 | — |
| 3# | Before hot-rolling | 1.80 | 77.5 | 64 | 13.80 | 3.0/5.0 | — |
| After hot-rolling | 73.5 | 61 | 12.78 | 3.0/5.0 | 8.4 | ||
| Before hot-rolling | 2.00 | 84.0 | 70 | 14.31 | 3.5/6.5 | — | |
| After hot-rolling | 78.0 | 68 | 10.22 | 3.0/6.5 | 7.7 | ||
| Before hot-rolling | 2.20 | 91.5 | 77 | 14.82 | 5.0/7.0 | — | |
| After hot-rolling | 81.5 | 71 | 10.75 | 4.5/6.5 | 7.1 |
Note: hot-rolling temperature was 150 °C; test conditions of high-temperature high-pressure fluid loss were 150 °C and 3.5 MPa; “—” means untested.
6. Conclusions
AM-AMPS-TAC polymers with different charge distributions were synthesized with AM, AMPS and TAC by changing feed ratios of the monomers. These polymers have salt- responsiveness of various degrees, and the closer the numbers of AMPS structure units and TAC structure units in the polymer chain, the more significant the salt-responsiveness of the polymer will be. Mechanism research indicates that the salt-responsive behavior of AM-AMPS-TAC polymer derives from extension of chain conformation caused by the weakening of intramolecular ionic bonds. Among all AM-AMPS- TAC polymers, AM90-AMPS5-TAC5 with neutrally electric chain has the best compatibility with salt, and can keep bentonite base mud saturated with NaCl stable in viscosity and in good dispersion for long time under high temperature. Saturated saltwater drilling fluid prepared with AM90-AMPS5- TAC5 has good basic properties.
The results can provide guidance for designing molecular structure of salt-tolerant polymer additive, extending applications of stimulus-responsive polymers in drilling fluid and developing drilling fluids with self-adaptability to environments in the future.
Nomenclature
C—solution concentration, g/L;
CNaCl—NaCl solution concentration, mol/L;
m, n, o—numbers of AMPS, AM and TAC structure units in polymer chain;
nAMPS and nTAC—amount of substance of AMPS and TAC structure units in polymers, mol;
Sc, Sh—areas of peak c and peak h, dimensionless;
β—Kramer constant;
δ—chemical shift, dimensionless;
κ—Huggins constant;
η—apparent viscosity, mPa•s;
ηin—intrinsic viscosity, L/g;
ηr—relative viscosity, dimensionless;
ηsp—specific viscosity, dimensionless;
η0—solvent viscosity, mPa•s;
Ф6—reading of Fann viscometer at the speed of 6 r/min, dimensionless;
Ф3—reading of Fann viscometer at the speed of 3 r/min, dimensionless.
Reference
Emerging applications of stimuli-responsive polymer materials
URL PMID:20094081 [Cited within: 1]
Insights into a greener stimuli-responsive fracturing fluid for geothermal energy recovery
pH-responsive water-based drilling fluids containing bentonite and chitin nanocrystals
Super-amphiphobic, strong self-cleaning and high-efficiency water- based drilling fluids
The use of a pH-triggered polymer gelant to seal cement fractures in wells
Responsive stabilization of nanoparticles for extreme salinity and high-temperature reservoir applications
URL PMID:26278070 [Cited within: 3]
Electrolyte- and pH-responsive polyampholytes with potential as viscosity-control agents in enhanced petroleum recovery
Twenty items of new technology for oil and gas exploration and development in next decade
Research and application of a novel high temperature filter loss reducer
Poly (sodium p-styrene sulfonate) modified Fe3O4 nanoparticles as effective additives in water-based drilling fluids
A saturated saltwater drilling fluid based on salt-responsive polyampholytes
Viscosimeter on a microfluidic chip
URL PMID:16800711 [Cited within: 1]
Shear thinning in dilute polymer solutions
URL PMID:17129166 [Cited within: 1]
Synthesis and property evaluation of a salt- and alkali-resistant star-polymer