Introduction
For over a century, water-flooding has been one of the most successful and efficient secondary oil recovery method and can be implemented in the early or late stage of oilfield development[1,2,3]. Researches in recent years show waterflooding effect is strongly affected by the salinity and composition of the injected water. Quite a number of researchers have confirmed that compared with injecting sea water, smart water flooding, that is injecting sea water with modified salinity and ion composition can affect interactions between oil, brine and rock, enhance the microscopic displacement efficiency and finally oil recovery of carbonate reservoirs[1,2,3,4,5,6,7,8,9,10,11,12,13]. The study of Zhang et al.[14] showed that increasing the concentration of SO42- could improve the wettability of chalk layers. The research of Fathi et al.[9] showed that the ultimate oil recovery improved dramatically as the concentration of SO42- in the sodium chloride-free seawater increased to 4 times that of ordinary seawater. Chandrasekhar and Mohanty et al.[15] found that modified seawater containing Mg2+ and SO42- could change wettability of calcite and make calcite more water-wet. Awolayo et al.[6] carried out a series of tests on Middle East carbonate core plugs and observed that increasing the concentration of sulfate in the seawater could make the crude oil/brine/rock system less oil-wet. Apparently, injecting smart water can change the wettability of carbonate rock, making it shift from oil-wet condition to water-wet condition, and thereby enhancing oil recovery. But if the injected smart water is poorly compatible with the original formation water, minerals could precipitate to form scale, which in turn could plug the matrix pores or fractures in oil-bearing formations, perforation channels, and down hole equipment, leading to decrease of oil and gas production at last[16,17,18,19,20,21,22]. The most common mineral scales in oilfields are divided into two categories, sulfate and carbonate scales. After injection, the SO42- in the smart water would react with the Ca2+ in the formation water to form CaSO4[23,24,25,26]; the CO32- in the smart water would react with the Ca2+ in the formation water to form CaCO3[27,28]. Generally, the formation water of carbonate reservoir has high salinity and high Ca2+ concentration. The mixing of smart water with high sulfate concentration and formation water would lead to the formation of CaSO4 scale and much smaller amount of CaCO3 scale. The CaSO4 scale is a main kind of scale in oil industry that can bring major troubles.
Researchers have conducted some experiments to find out the impact of in-situ mineral scale formation on reservoir permeability. Moghadasi et al.[23] found through experiments that the permeability of reservoirs after water injection could drop by less than 30% to more than 90% due to the effect of CaCO3 or CaSO4 scale; the degree of permeability decline depended on the composition of the formation water and injected water, initial permeability and temperature of the reservoir, water injection duration and rate etc. Bin Merdhah and Yassin[25] performed core flooding tests to study the permeability decline caused by common oil field scales formed in the sandstone cores when injected sea water and formation water with high concentration of barium, strontium, and calcium ions mixed in different conditions. The results showed that the CaSO4 and SrSO4 scale increased as the temperature rose, but BaSO4 scale decreased because the solubility of BaSO4 scale increased with the rise of temperature; all of them increased at higher differential pressure and higher salinity of injected water and formation water. Tahmasbi et al.[24] investigated the permeability decline of packed columns of carbonate grain and glass bead when injected waters with high concentrations of calcium and sulfate ions mixed. The results showed that with the increase of concentrations of these ions in the injected water, the CaSO4 precipitation increased, and the permeability of the packed columns dropped continuously; the permeability reduction rate didn’t decrease with the increase of the injection rate; at a given concentration and injection rate, the rate of permeability decline became faster with the rise of temperature. Moghadasi et al.[29] found that in-situ scale precipitation would result in significant drop of permeability of sandstone cores; the calcium sulfate and strontium sulfate scales increased with the rise of temperature, but the precipitation of barium scale decreased; and the precipitation of all of them increased with the rise of pressure. Naseri et al.[30] found through study that the composite BaSO4-CaSO4 scaling was opposite to the BaSO4 scaling, the higher the temperature, the more the amount of composite BaSO4-CaSO4 scale, and the more rapidly the permeability of porous media would decrease. It can be seen that factors affecting mineral scale and consequently permeability include temperature, pressure, type of scale, salinity of injected and formation waters, water injection rate and initial reservoir permeability etc. Based on published researches, most of the previous smart water flooding plans focused on EOR only but paid little attention to the formation damage and reduction in the productivity of reservoir caused by water injection.
We carried out[31] static compatibility tests of mixing brines with different compositions at the atmospheric pressure and the temperature of 90 °C to find out the impact of salinity and ionic concentration of smart water on CaSO4 scaling by removing the HCO3- in sea water to simplify the sea water composition. In this work, core flooding experiments were carried out under newly designed water injection program and experimental parameters to model the reaction of smart water and original formation water in carbonate reservoir to find the effect of scaling on reservoir permeability more accurately. Experiments were carried out in this work to study risk of scaling when smart water was used as injected water to enhance oil recovery, how to lower the risk of scaling by adjusting the salinity and ion composition of the smart water and the reduction of permeability caused by composite CaSO4 and CaCO3 scaling, and analyze the crystal size and morphology of composite CaSO4-CaCO3 scale on the rock pore surface by SEM photographs.
1. Materials and procedures
1.1. Brines
Six kinds of high purity analytical grade salts provided by Merck company, including NaCl, KCl, Na2SO4, NaHCO3, MgCl2•6H2O and CaCl2•2H2O were used to prepare the synthetic brine solutions. The predetermined amounts of the salts were dissolved in deionized water to prepare the brine solutions. The brines were stirred by a magnetic stirrer and set aside for 24 hours to ensure that the brines were stable. Also to ensure that there were no suspended particles in the solution, the brines were filtered with 0.45 μm filter paper. The synthetic Persian Gulf seawater (PGSW) and the synthetic Koupal field formation water (KFW) were prepared by the above preparation method, and their chemical compositions are shown in Table 1. Modified brines were made based on the composition of Persian Gulf seawater to study the composite CaSO4 and CaCO3 scale formed at different salinities and ion compositions of smart water and the effect of the scale on reservoir permeability. Two sets of modified brines were prepared to study the effect of salinity and ionic composition of the injected smart water on the permeability reduction caused by the composite CaSO4 and CaCO3 scale. One set of modified brines with constant salinity (Table 2) was used to study the effect of ionic composition of injected smart water of scaling. The PGSW_1Ca1Mg2S means the concentrations of Ca2+, Mg2+ and SO42- of the modified brine are 1, 1 and 2 times those of the PGSW, and so on and so forth. The other set of modified brines (Table 3) was prepared by diluting the seawater to study the effect of injected water salinity on scaling. PGSW#5D means the PGSW is diluted 5 times, and so on so forth.
Table 1 Chemical composition of PGSW and KFW.
| Brine | Ion concentration/(mg·L-1) | Ionic strength/ (mol·L-1) | pH | TDS/ (mg·L-1) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Na+ | Cl- | K+ | Ca2+ | Mg2+ | SO42- | HCO3- | ||||
| KFW | 41 350 | 92 785 | 15 200 | 729 | 3.026 | 8.16 | 150 064 | |||
| PGSW | 12 653 | 22 598 | 420 | 498 | 1 408 | 3 037 | 73 | 0.833 | 7.47 | 40 687 |
Table 2 Chemical composition of smart water with different ion compositions.
| Brine | Ion concentration/(mg·L-1) | Ion strength/ (mol·L-1) | pH value | TDS/ (mg·L-1) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Na+ | Cl- | K+ | Ca2+ | Mg2+ | SO42- | HCO3- | ||||
| PGSW | 12 653 | 22 598 | 420 | 498 | 1 408 | 3 037 | 73 | 0.833 | 7.47 | 40 687 |
| PGSW_1Ca1Mg2S | 12 340 | 19 875 | 420 | 498 | 1 408 | 6 073 | 73 | 0.851 | 7.52 | 40 687 |
| PGSW_1Ca3Mg1S | 8 313 | 24 122 | 420 | 498 | 4 224 | 3 037 | 73 | 1.018 | 7.49 | 40 687 |
| PGSW_1Ca4Mg1S | 6 143 | 24 884 | 420 | 498 | 5 632 | 3 037 | 73 | 1.058 | 7.51 | 40 687 |
Table 3 Chemical composition of smart water with different salinities.
| Brine | Ion concentration/(mg·L-1) | Ion strength/ (mol·L-1) | pH | TDS/ (mg·L-1) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Na+ | Cl- | K+ | Ca2+ | Mg2+ | SO42- | HCO3- | ||||
| PGSW | 12653 | 22598 | 420 | 498 | 1408 | 3037 | 73 | 0.833 | 7.47 | 40,687 |
| PGSW # 5D | 2531 | 4520 | 84 | 100 | 282 | 607 | 14.5 | 0.167 | 6.87 | 8137 |
| PGSW # 10D | 1265 | 2260 | 42 | 50 | 141 | 304 | 7 | 0.083 | 6.85 | 4069 |
1.2. Core material
All the core samples were taken from the carbonate dolomite outcrop in the Babakoohi mountain of Shiraz, which were low in clay content. The core samples were 7 cm in length, 3.8 cm in diameter, 13.61% in average porosity and 33.37×10-3 μm2 in absolute permeability. All the core samples were cleaned at ambient temperature by toluene in a Soxhlet extractor, and dried in an oven at 100°C for 24 hours before use. The physical properties of all the core samples are listed in Table 4. X-ray diffraction (XRD) analysis of the rock core samples shows that the core samples have more than 99% of dolomite particles, as shown in Fig. 1.
Table 4 Physical properties of core samples used in this research
| Serial No. | Length/ cm | Diameter/ cm | Pore volume/ cm3 | Porosity/ % | Absolute permeability/ 10-3 μm2 |
|---|---|---|---|---|---|
| 1 | 7 | 3.8 | 11.09 | 13.98 | 33.04 |
| 2 | 7 | 3.8 | 10.21 | 14.13 | 35.6 |
| 3 | 7 | 3.8 | 10.87 | 13.7 | 33.53 |
| 4 | 7 | 3.8 | 10.64 | 13.41 | 34.19 |
| 5 | 7 | 3.8 | 10.68 | 13.46 | 34.44 |
| 6 | 7 | 3.8 | 10.72 | 13.51 | 35.13 |
| 7 | 7 | 3.8 | 10.66 | 13.43 | 34.69 |
| 8 | 7 | 3.8 | 10.65 | 13.42 | 29.1 |
| 9 | 7 | 3.8 | 10.66 | 13.42 | 30.59 |
Fig. 1.
Fig. 1.
XRD pattern of a carbonate core sample. n—counts; θ—diffraction angle.
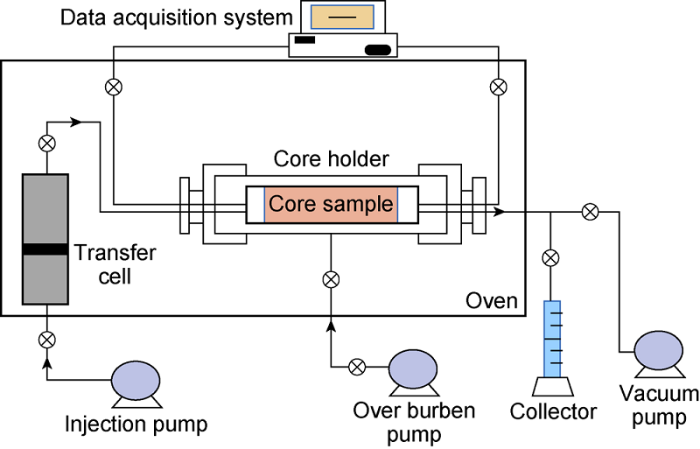
Fig. 2 shows the core flooding apparatus. Before each core-flooding run, the core sample was first saturated with KFW and then the pore volume and initial porosity were calculated. Thereafter, the saturated core was flooded with KFW at variable flow rates and the corresponding differential pressures were recorded, the initial core permeability was calculated with Darcy’s Law, and the results are given in Table 4.
Fig. 2.
Fig. 2.
Schematic diagram of the core-flooding apparatus.
After each core-flooding test, the used core was restored and prepared for the next core-flooding as follows. (a) If the rock core sample contained a small amount of clay: (1) the core was flooded with 6 PV of distilled water; (2) followed by 2PV of toluene to completely clean the rock core sample; (3) finally, the core sample was dried in an Oven at 100 °C for 24 hours to be prepared for the next core-flooding run. (b) If the rock core sample contained a high percentage of clay: (1) the rock core sample was flooded with 6 PV of saline water without SO42- and CO32- ions; (2) followed by 2PV of toluene to completely clean the core; (3) finally, the core sample was dried in an Oven at 100 °C for 24 hours to be prepared for the next core-flooding test[32].
1.3. Procedure of core-flooding tests
Three groups of core-flooding experiments were conducted under normal pressure at 90 °C. In the first group, the synthetic PGSW was used to displace the core saturated with KFW to test the extent of permeability decline caused by composite CaSO4 and CaCO3 scale. The second group of core-flooding experiments were performed to investigate the effect of contact time (CT) of injected water and formation water in the porous media on the scale quality. CT was a new parameter designed in this work. The third group of core-flooding experiments were conducted to study the salinity and ionic composition of injected PGSW on the permeability decline due to formation of in-situ composite CaSO4-CaCO3 scale. Finally, in order to study the morphology of deposited scale on the rock pore surface, the SEM photographs of scaled core samples were analyzed visually.
A new experiment procedure was designed to model the flow of injected water and formation water in the real reservoir conditions. After the porosity and initial core permeability were measured by injection KFW into the core, the transfer cell containing KFW was switched to the transfer cell of smart water and placed inside the oven together with the core holder assembly, and heated to the temperature of 90 °C. The system was left for 4-5 hours to reach the desired temperature. All core-flooding experiments were carried out at a constant flow rate by setting the injection pumping rate at 0.5 mL/min. First, 2PV of the smart water was injected into the core sample. After the water injection, the contact time was set to leave the incompatible injected water and formation water to contact and react with each other fully. Afterward, 3PV of smart water was injected into the core sample and the differential pressure between the two ends of core sample was recorded continuously. The final permeability of core sample was calculated using Darcy's linear-flow equation after scale formation. After flooding, the scaled rock core sample was taken out, dried in an oven at 100°C and cut into smaller pieces for SEM analysis.
1.4. Prediction of scaling potential
The thermodynamics of mineral scaling by mixing the sulfate-rich PGSW and KFW was first analyzed with the OLI ScaleChem software to find out the scaling minerals under the experimental conditions in this work. A chemistry simulation program was developed based on thermodynamic balance and solubility of ions in water to predict scaling during water injection in the reservoir[22].
2. Results and discussion
2.1. Prediction and analysis of mineral-scaling potential
The OLI ScaleChem software was used to predict the type of scale could be formed, to calculate the scaling potential and the amount of precipitated scale at the temperature of 90 °C and atmospheric pressure. The chemical compositions of the mixed brines used to predict the potential of mineral scale formation are shown in Table 1. The potential of scaling was evaluated by the scaling trend (ST), when the ST is greater than 1.0, the scaling potential is high.
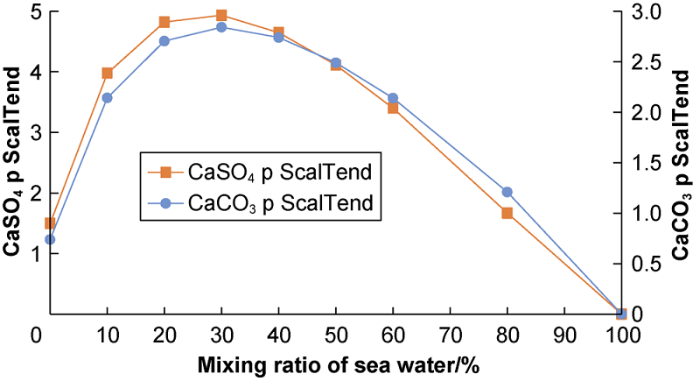
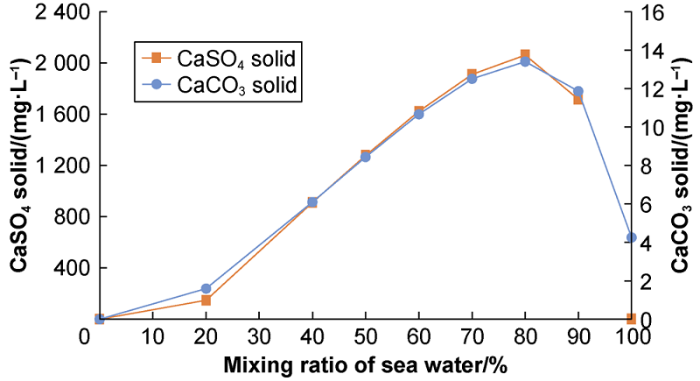
Fig. 3 shows the potential scaling tendency as a function of PGSW ratio. The ratio of PGSW is the ratio of PGSW in the mixed solution in volume, for example, the ratio of PGSW is 80% means in 100 L of the mixed solution, there is 80 mL PGSW and 20 mL of KFW. The results show the maximum ST of CaSO4 and CaCO3 are 4.9 and 2.8 respectively. Clearly, the CaSO4 scaling is higher. Fig. 4 shows the amount of scale formed as a function of ratio of PGSW in the case formation water and seawater are mixed. The results show the amount of calcium sulfate scale was the maximum of 2060.9 mg/L and the amount of calcium carbonate was the maximum of 13.4 mg/L at 80% PGSW. Calcium sulfate is the main type of scale in the smart water flooding system described herein.
Fig. 3.
Fig. 3.
The potential of scaling tendency with ratio of PGSW at 90 °C and 1 atm.
Fig. 4.
Fig. 4.
The amount of scale with ratio of PGSW at 90 °C and 1 atm.
2.2. Core tests
All the core-flooding experiments were carried out with single phase fluid at a constant flow rate of 0.5 mL/min. The final permeability of the core sample was calculated with the recorded differential pressure data by Darcy's linear-flow equation. The permeability ratio was then plotted vs. pore volume of injected brine. The experimental results are shown in Table 5. Finally, the scaled core sample was observed visually by SEM to find out the crystal size and morphology of composite CaSO4-CaCO3 scale on the rock pore surface.
Table 5 Statistics on experimental results on core samples.
| No. | Contact time/ h | Injected water | Initial permeability/ 10-3 μm2 | Final permeability/ 10-3 μm2 | Extent of permeability drop/% |
|---|---|---|---|---|---|
| 1 | 0 | PGSW | 33.04 | 12.93 | 60.86 |
| 2 | 12 | PGSW | 35.60 | 9.20 | 74.16 |
| 3 | 24 | PGSW | 33.53 | 7.81 | 76.71 |
| 4 | 48 | PGSW | 34.19 | 7.92 | 76.84 |
| 5 | 12 | PGSW_1Ca3Mg1S | 34.44 | 9.94 | 71.14 |
| 6 | 12 | PGSW_1Ca4Mg1S | 35.13 | 11.51 | 67.24 |
| 7 | 12 | PGSW_1Ca1Mg2S | 30.59 | 6.39 | 79.10 |
| 8 | 12 | PGSW#5D | 34.69 | 11.26 | 67.54 |
| 9 | 12 | PGSW#10D | 29.10 | 8.38 | 71.20 |
Note: All the experiments were done at 90 °C and normal pressure, all the scale was composite CaSO4 and CaCO3, the formation water was KFW, the water injection rate was 0.5 mL/min constantly.
2.2.1. Degree of permeability damage
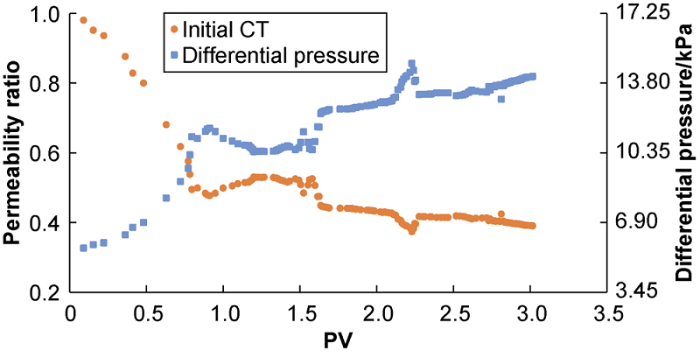
The synthetic PGSW was injected into core sample saturated with KFW to study the degree of permeability reduction caused by the composite CaSO4 and CaCO3 scale. In this test, the contact time was 0 h, that is initial contact, corresponding to the experiment No.1 in Table 5.
Fig. 5 shows the permeability of the core decreased by about 61% due to the formation of CaSO4-CaCO3 scale. The permeability decline trend changed with the pore volume of injected PGSW. During the initial period of PGSW injection (one pore volume injected), the permeability decreased sharply. Then the decrease rate of permeability slowed down, and permeability gradually became stable after the sharp drop. This is because in the early period of PGSW injection, the composite CaSO4- CaCO3 became saturated fast and then precipitated in the rock pores into scale, blocking the pores.
Fig. 5.
Fig. 5.
Variation curves of ratio of final permeability to initial permeability and differential pressure with injected PGSW volume (experiment # 1).
After the nucleation of CaSO4 and CaCO3 scale, at the beginning, the permeability reduction is mostly caused by crystal growth. Later, as crystals deposit on the pore surface continuously, scale occupies more and more pore spaces. Crystal growth and crystal deposition are slower than scale nucleation. Thus, the reservoir damage includes three stages, scale nucleation (or precipitation), crystal growth, and in-situ crystal deposition[33].
Fig. 5 shows that the permeability drop has fluctuations. This is because several suspended scale particles may bridge across a pore throat, and a single scale particle may block a pore throat. The blocking and bridging of pore throat by suspended scale particles can cause the differential pressure on two side of pore throat to increase, until the particles passing the pore throat and the bridge break by supplied pressure, and consequently the differential pressure decreases. This phenomenon occurs repeatedly during the injection of PGSW[15].
2.2.2. Effect of CT on scale precipitation
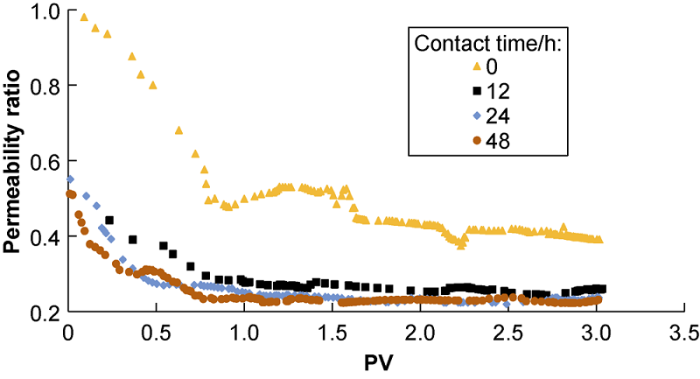
As mentioned above, the experiment was conducted to find out the CT between injected water and formation water when the amount of composite CaSO4-CaCO3 scale reached the maximum. To be specific, four experiments at the contact time of 0, 12, 24, and 48 hours were performed, corresponding to core-flooding runs 1, 2, 3, and 4, respectively in Table 5. In these experiments, 2PV of PGSW was injected first, after reaching the set contact time, more PGSW was injected and the pressure difference of the two ends of the core sample was recorded to calculate the final permeability. Fig. 6 shows with the increase of contact time, the amount of composite CaSO4 and CaCO3 scale after mixing of PGSW and KFW increases and the ratio of final permeability to initial permeability decreases. After the injected water mixed with the formation water for 12 hours within the core, the composite CaSO4-CaCO3 scale had caused severe reservoir damage, leading to higher permeability loss. When the contact time extended to 24 h and 48 h, the permeability loss didn’t increase significantly. The next other core- flooding runs were all completed at the contact time of 12 hours.
Fig. 6.
Fig. 6.
The ratio of final permeability to initial permeability vs pore volume during injection of synthetic PGSW at different contact time, (corresponding to run # 1, 2, 3& 4).
2.2.3. Effect of ionic composition of injected water on scaling
The chemical composition of injection water is one of the most important factors affecting the scaling tendency. To examine the effect of ion composition of injected water on permeability decline, a group of core-flooding tests were carried out, namely experiments 1, 5, 6, and 7 in Table 5. The chemical compositions of PGSW are shown in Table 2.
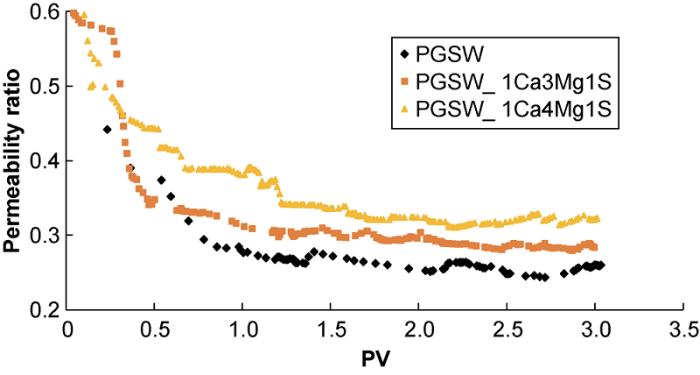
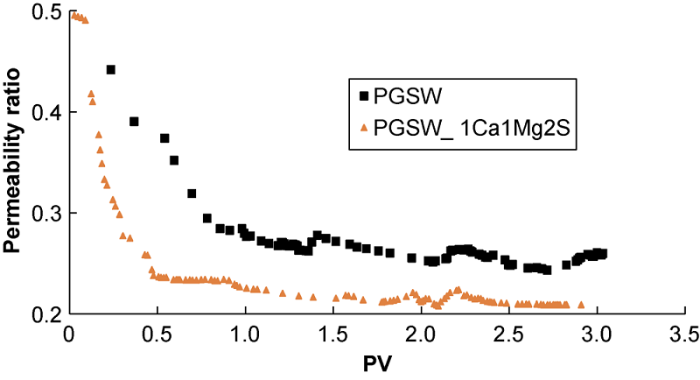
Fig. 7 shows the amount of composite CaSO4-CaCO3 scale decreases with the increase of Mg2+ concentration in the injected smart water, leading to the increase of the ratio of final permeability to initial permeability. Ions do not exist totally as free ions in the solution, and ion pairing in the solution is very complex. If bound ions are not available for scale formation and thus they increase the solubility of mineral salts by decreasing the reactants’ available concentration[31]. In these core-flooding tests, the chemical composition of the injected brines contains magnesium ion, sulfate ion, and carbonate ion, and the magnesium ion can make complex or undergo ion pairing with sulfate ion and carbonate ion, lowering the concentrations of sulfate ion and carbonate ion, so less composite CaSO4-CaCO3 scale will be formed.
Fig. 7.
Fig. 7.
The ratio of final permeability to initial permeability vs. pore volume during injection of smart water with changed magnesium ion concentration and ordinary seawater (corresponding to run # 1, 5, and 6).
Fig. 8 shows with the increase of sulfate ion concentration in the smart water, the amount of composite CaSO4-CaCO3 scale formed increases, and the ratio of final permeability to initial permeability decreases. This is because super-saturation increases dramatically when the concentration of sulfate ion in the smart water exceeds that in PGSW, reactivity speed of the sulfate ions with calcium ions increases as well, and the amount of calcium sulfate scale formed increases, leading to severe reservoir damage and permeability loss.
Fig. 8.
Fig. 8.
The ratio of final permeability to initial permeability vs. pore volume of injected smart water and ordinary seawater (corresponding to run # 1, and 7).
2.2.4. Effect of salinity of injected water on scaling
Previous laboratory studies showed that injecting low salinity brine had a significant effect on oil recovery[1, 8, 26]. To investigate the potential damage of injecting low salinity brine, a group of experiments were designed, namely, experiments 1, 8 and 9 in Table 5. The chemical composition of the injected water in the experiments is shown in Table 3.
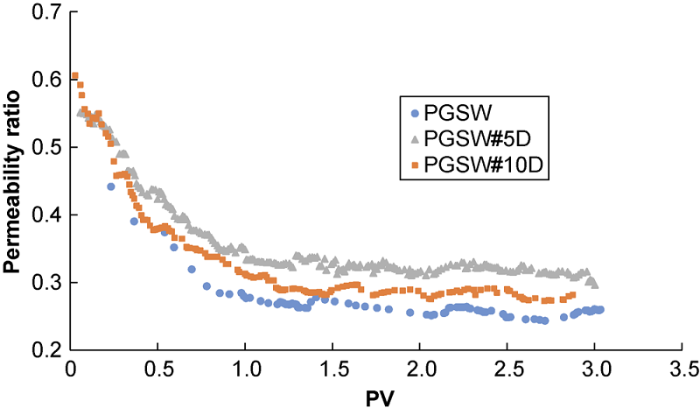
Fig. 9 shows changing the injected water salinity has significant impact on the amount of scale formed and in turn permeability. The salinity of injected fluid can be lowered by dilution. When the PGSW was diluted 5 times, the ratio of final permeability to initial permeability increased to some extent, but with further dilution, the ratio decreased. Through an earlier study[31], we found that the salinity and concentration of active ions of solution controlled the solubility of mineral salts. Salinity has impact on scale precipitation opposite to the concentration of active ions. The amount of scale precipitated decreases first and then increases with the increase of the salinity of solution, and there is an optimum salinity (PGSW diluted 5 times under the experimental condition in this work) at which the mineral salts have the highest solubility. The concentrations of reagent ions such as Ca2+, CO32- and SO42- in the solution reduced by diluting PGSW 5 times with deionized water (10% salinity of seawater), so the activity product of reagent ions decreased. In addition, at this salinity (5 time diluted PGSW), the salinity of injected PGSW is still the effective parameter compared with the concentration of reagent ions, and leads to a reduction in the composite CaSO4-CaCO3 scale formed. However, when the PGSW is further diluted (i.e. 10 times), the effects of salinity and concentration of reagent ions will reverse, the activity product of reagent ions will increase, and the activity of anions and cations will enhance, resulting in the formation of more composite CaSO4-CaCO3 scale, and in turn reduction of permeability.
Fig. 9.
Fig. 9.
Ratio of final permeability to initial permeability vs. pore volumes of ordinary seawater and smart water (corresponding to experiments # 1, 8, and 9).
2.2.5. Scanning electron microscopy (SEM) analysis
SEM was used to study the shape and particle size, deposition sites, and distribution of scale crystals on the rock pore surface. Take the experiment # 7, the core sample with scale was removed at the end of water-flooding, dried and cut into smaller sections for SEM analysis.
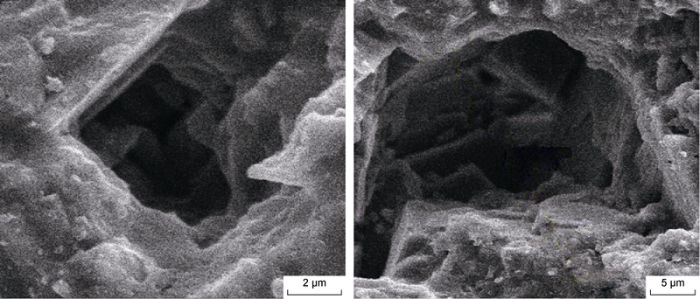
Fig. 10 shows the SEM images of the core sample before scaling. Fig. 11 shows the SEM image of the composite CaSO4-CaCO3 scale crystals in the rock pores, it can be seen some crystals gather and are perpendicular to the pore surface. Compared with scale crystals on the rock surface, scale crystals perpendicular to the pore surface have greater resistance to flow of formation water. Therefore, the permeability loss of core samples is mainly caused by these crystals. The scale crystals are irregular in shape, some are tabular and some plate-like. They deposit on top of each other and grow perpendicular to the pore wall. Also, both single and conglomeration of crystals are observed.
Fig. 10.
Fig. 10.
SEM images of a carbonate dolomite core before scaling.
Fig. 11.
Fig. 11.
SEM images of composite CaSO4-CaCO3 scale crystals in dolomite core.
3. Conclusions
When there are bivalent ions in the formation water of carbonate reservoir, injecting smart water has the risk of scaling. The salinity and ionic composition of the injected water have strong impacts on the extent of permeability loss. Precipitation of composite CaSO4-CaCO3 scale is the main cause of permeability decline of carbonate reservoir. The permeability decline caused by composite CaSO4- CaCO3 scale formation in the porous media ranged from 61% to 79.1% of the initial permeability under the experimental conditions in this work. The extent of permeability decline depends on the ion composition and salinity of the injected water, and contact time of the injected water and formation water.
The results show that increasing the magnesium ions concentration or decreasing SO42- concentration of the injected smart water can reduce the amount of composite CaSO4-CaCO3 scale formed, and in turn the permeability loss.
Changing the salinity of injected seawater has a significant effect on the scale precipitation in porous media, and in turn on the permeability decline. The salinity of seawater can be reduced through dilution. When the seawater was diluted 5 times, the ratio of final permeability to initial permeability increased somewhat, but the ratio decreased with further dilution.
SEM images confirm composite CaSO4-CaCO3 deposits and grows on the rock pore surface, and the composite CaSO4-CaCO3 scale crystals often grow perpendicular to the pore wall. The permeability loss of core is mainly caused by crystals growing perpendicular to the pore wall.
Acknowledgments
The authors thank the “Institute of Petroleum Engineering in University of Tehran”, for all their support in this investigation.
Reference
Mechanistic modeling of low salinity water injection
Mechanisms behind low salinity water injection in carbonate reservoirs
Laboratory investigation of novel oil recovery method for carbonate reservoirs
Wettability alteration and improved oil recovery by spontaneous imbibition of seawater into chalk: Impact of the potential determining ions Ca2+, Mg2+, and SO42-
Low-salinity flooding in a selected carbonate reservoir: Experimental approach
A laboratory study of ionic effect of smart water for enhancing oil recovery in carbonate reservoirs
Review of recovery mechanisms of ionically modified waterflood in carbonate reservoirs
From mineral surfaces and coreflood experiments to reservoir implementations: Comprehensive review of low-salinity water flooding (LSWF)
Water-based enhanced oil recovery (EOR) by “smart water”: Optimal ionic composition for EOR in carbonates
Role of divalent ions, temperature, and crude oil during water injection into dolomitic carbonate oil reservoirs
Effect of water salinity on oil/brine interfacial behaviour during low salinity waterflooding: A mechanistic study
DOI:10.1016/j.petlm.2019.03.005 URL [Cited within: 1]
Experimental investigation of the influence of fluid-fluid interactions on oil recovery during low salinity water flooding
DOI:10.1016/j.petrol.2019.106194 URL [Cited within: 1]
Smartwater flooding in a carbonate asphaltenic fractured oil reservoir: Comprehensive fluid-fluid-rock mechanistic study
Wettability and oil recovery from carbonates: Effects of temperature and potential determining ions
DOI:10.1016/j.colsurfa.2006.01.009 URL [Cited within: 1]
Wettability alteration with brine composition in high temperature carbonate reservoirs
Laboratory and prediction of barium sulfate scaling at high-barium formation water
DOI:10.1016/j.petrol.2009.10.001 URL [Cited within: 1]
Mechanisms of scale deposition and scale removal in porous media
DOI:10.1504/IJOGCT.2008.016733 URL [Cited within: 1]
Scaling problems in western Siberia
Scale formation in Iranian oil reservoir and production equipment during water injection
Implications of brine mixing in the reservoir for scale management in the alba field
Examination of the effect of generically different scale inhibitor species (PPCA and DETPMP) on the adherence and growth of barium sulphate scale on metal surfaces
Prediction of scale formation problems in oil reservoirs and production equipment due to injection of incompatible waters
DOI:10.1002/apj.5500140319 URL [Cited within: 2]
Scale formation in oil reservoir and production equipment during water injection: Kinetics of CaSO4 and CaCO3 crystal growth and effect on formation damage
Dimensionless correlation for the prediction of permeability reduction rate due to calcium sulphate scale deposition in carbonate grain packed column
DOI:10.1016/j.jtice.2009.11.006
URL
[Cited within: 2]

Abstract
In this work, an experimental and theoretical study has been conducted to investigate the permeability reduction due to CaSO4 scale deposition in packed column porous media. Permeability reduction by calcium sulphate deposition follows a systematic trend considering various important parameters that are affected in this complex process. Hence, a novel dimensionless model has been proposed for the prediction of permeability reduction rate with high accuracy. The developed model is based on the data obtained from glass bead and carbonate grain packed column at low pressure. The proposed model was validated with Berea sandstone cores data at high pressure (100–20,678 kPa), various flow rates (5–15 cm3/min), different temperatures (45–95 °C) and various concentrations of calcium and sulphate ions in brine solutions (0.0125–0.0225 mol/L).
Formation damage due to scale formation in porous media resulting water injection
DOI:10.1016/0346-251X(86)90046-1 URL [Cited within: 2]
Experimental investigation of water incompatibility and rock/fluid and fluid/fluid interactions in the absence and presence of scale inhibitors
Scale prediction for oil and gas production
DOI:10.2118/132237-PA URL [Cited within: 1]
The use of flow models in assessing the risk of scale damage
Experimental investigation of prediction and inhibition of sulfate scales
Effect of temperature and calcium ion concentration on permeability reduction due to composite barium and calcium sulfate precipitation in porous media
DOI:10.1016/j.jngse.2014.12.007 URL [Cited within: 1]
Effect of salinity and ion type on formation damage due to inorganic scale deposition and introducing optimum salinity
Determination of in-situ precipitation of barium sulphate during coreflooding
Barium and strontium sulfate solid solution formation in relation to north sea scaling problems