Introduction
The chemical reactions in the process of CO2 flooding can change the physical properties of the reservoir, thereby affecting the oil recovery[1,2,3,4,5]. A large number of research results show that the CO2 injected into the formation during the CO2 flooding process will dissolve in the formation water to produce carbonate precipitation, which will block the rock pore throat, and make the porosity and permeability of the reservoir and thus the oil recovery factor reduce[6,7,8,9]. Temperature, pressure, and the mass concentration of scale-forming ions in formation water are the important factors affecting the inorganic salt precipitation caused by interaction of CO2 with formation water.
Ross et al.[10] conducted CO2-formation water-rock interaction experiments on calcareous sandstone samples from the North Sea Oilfield in the United Kingdom and found that the core samples had much lower permeability after the experiment than before the experiment. Xiao et al.[11] conducted CO2-water-calcite immersion experiments under different conditions, and found that with the increase of the experimental pressure, the porosity of the rock first increased and then decreased. Zeidouni et al.[12] and Sbai et al.[13] simulated the salt precipitation in formation water in the process of CO2 flooding and found that the porosity of the reservoir near the borehole decreased due to salt precipitation.
The Chang 8 block of the Changqing oilfield is a typical tight oil reservoir, and the formation fluid has high salinity and calcium ion concentration. During the CO2 flooding, the CO2-water-rock interaction caused damage to the reservoir, severely impairing oil field production. It is necessary to study the formation and distribution of inorganic salt precipitation during CO2 flooding. At present, most researchers mainly focus on the qualitative study of CO2-water-rock interaction, and have not quantified the amount of precipitate under different conditions and the impact of precipitate on the physical properties of the reservoir. In view of the aforementioned problems, CO2-formation water interaction static immersion experiments and dynamic displacement experiments under different pressure differences, different temperatures, and different scale ion mass concentrations were performed in this work on the basis of previous research results. A quantitative mathematical characterization equation for the amount of sediment produced by the interaction of CO2-formation water and a mathematical characterization equation for the effect of sedimentation on the physical properties of the reservoir were established. The mathematical characterization equation was used to modify the numerical simulation model of the Eclipse numerical simulation software E300 module. On this basis, the distribution law of inorganic salt precipitate generated during the CO2 flooding in the Chang 8 block of the Changqing oilfield was studied, and the effect of inorganic salt precipitation on oilfield recovery was predicted.
1. Measurement of precipitate amount
In the experiments, the water samples used was formation water samples from 3 oil wells in the Chang 8 block of the Changqing oilfield. The mass concentrations of main scale-forming ions in each water sample are shown in Table 1. Scaling ions in the water samples were mainly Ca2+, Mg2+, Ba2+, Sr2+, of which Ca2+ content is more than 95%, and the Mg2+, Ba2+, Sr2+ contents were very low, and the water was CaCl2 type. The reservoir rocks in this block were mainly composed of feldspar minerals and quartz, and the feldspar mainly includes albite and potash feldspar. The core used in the experiment was also taken from the Chang 8 block of the Changqing oilfield, and had an average porosity of 10% and an average permeability of 0.1×10-3-0.4×10-3 μm2.
Table 1 Mass concentration of main scale-forming ions in the formation water samples.
| Formation water sample | Mass concentrations of main scale-forming ions/(mg·L-1) | Total salinity/ (mg·L-1) | |||
|---|---|---|---|---|---|
| Ca2+ | Mg2+ | Ba2+ | Sr2+ | ||
| 1 | 2012 | 198 | 0.006 | 19.4 | 16 090 |
| 2 | 5145 | 209 | 0.057 | 25.4 | 32 375 |
| 3 | 10590 | 222 | 0.028 | 18.8 | 57 000 |
1.1. Experimental mechanism and method
1.1.1. The mechanism of precipitation
CO2 dissolves in water to form H2CO3, and further ionizes to form CO32- and HCO3-. Under normal temperature conditions, only H2CO3 exists in acidic (pH<4.5) solutions; CO32- is mainly present in alkaline (8.34<pH<12.00) solutions; HCO3- is mainly present in neutral, slightly acidic and alkaline solutions, and there is no CO32-[14,15]. The process of CO2 and formation water interaction to generate sediment can be expressed as (1):
CaCO3 will precipitate and deposit only when it is saturated, so only when the ion content in the solution is very high, the CaCO3 crystal nucleus can be generated and precipitate[7]. According to the saturation index method of David-Stiff, the saturation index Is can be expressed as:
When Is≤0, CaCO3 in the water system does not precipitate; only when Is>0, CaCO3 will be oversaturated and precipitate in the water system.
1.1.2. Experimental method
1.1.2.1. Static experiment on CO2-formation water interaction
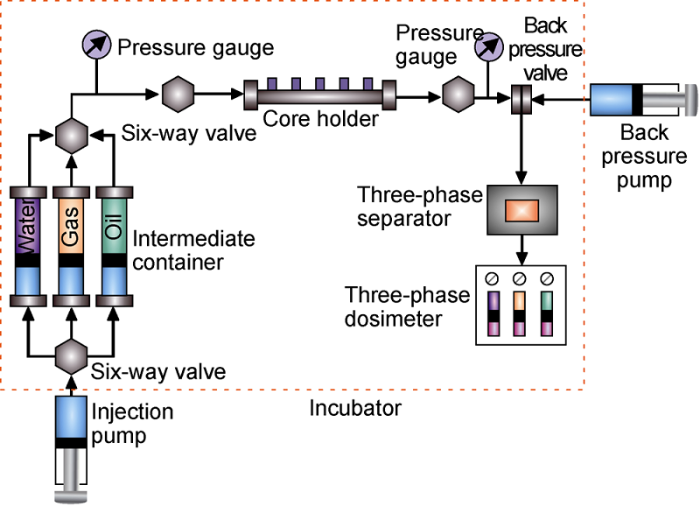
The laws of precipitates generated by CO2-formation water interaction under different temperatures (20, 30, 50, 80 °C) and different pressure differences (8, 10, 12, 16 MPa) were tested. (1) Enough CO2 was injected into the high temperature and high pressure reactor containing 100 mL of formation water; (2) ISCO pump was used to pressurize the reactor until its pressure stabilized at the required value; (3) the high-temperature and high-pressure reactor was put in a thermostat, at the designed temperature for 6 days; (4) after 6 days, ISCO pump was used to quickly reduce the pressure of the reactor to atmospheric pressure and the reactor should be set aside for 24 hours; (5) the high-temperature and high-pressure reactor was opened, and the solution was moved to another vessel to measure the ion concentrations with spectrometer, after that, the composition of the precipitate was analyzed by scanning electron microscope and energy spectrometer (EDS), the amount of precipitate was measured; (6) the experiment was repeated to test the amounts of precipitate at different temperatures, pressure differences, and mass concentrations of scaling ions. The experimental device is shown in Fig. 1.
Fig. 1.
Fig. 1.
Schematic diagram of the experimental device (a) and schematic diagram of high temperature and high pressure reactor (b).
1.1.2.2. CO2 displacement experiment
To study the influence of sediments on core physical properties, experiments of CO2-distilled water-rock and CO2-formation water-rock interaction under different temperatures (20, 30, 50, 80 °C) and different displacement pressures (19, 20, 21, 22 MPa) were carried out. The crude oil used in the experiment was degassed crude oil from the Changqing oilfield. The specific steps are as follows: (1) The core was vacuumed and placed in a core holder, flooded with water to clean pore throats inside the core to remove impurities, then the core was vacuumed again and dried. (2) According to the characteristics of low permeability reservoir core, the vacuumed core was put into the high temperature and high pressure reactor, the formation water was injected (distilled water in the distilled water experiment group), the pressure of the reactor was increased to the formation pressure (21 MPa) and kept for 12 hours, then the core was put into the core holder to be saturated with the formation water (distilled water). (3) The crude oil valve was opened and oil was injected to displace water until no water flew out at the outlet to achieve the purpose of saturating the core with crude oil, and then the core was aged. (4) 8-12 hours later, CO2 flooding experiment was started, ISCO pump pressure was adjusted to the experimental displacement pressure, the outlet pressure was set at 16 MPa, and the displacement was carried out until no crude oil came out at the outlet. The core permeability and porosity were tested after the experiment. The experimental device is shown in Fig. 2.
Fig. 2.
Fig. 2.
Experimental device for core-flooding.
1.2. Experimental results
1.2.1. Static experiments
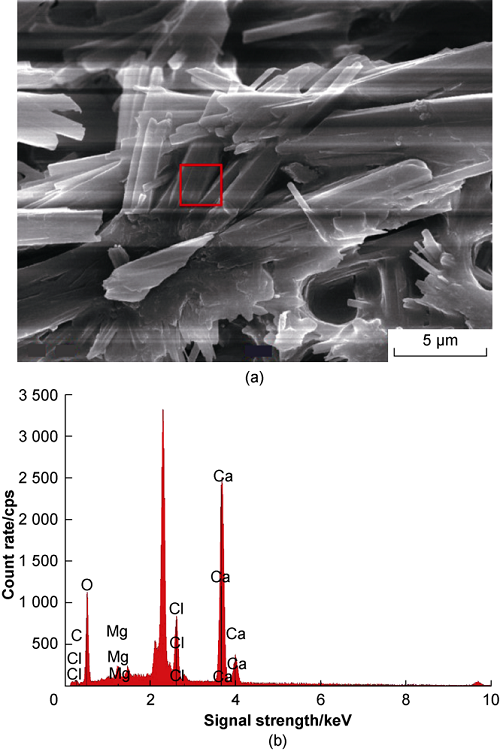
The resulting precipitates were filtered and dried, and then tested for element content by EDS. The measurement results are shown in Fig. 3 (the red box in Fig. 3a represents the scanning position corresponding to Fig. 3b). Based on the reaction formula of CO2 and formation water, it can be known that the inorganic salt precipitate produced is mainly CaCO3, and the precipitate minerals in the experiment also included CaCl2 and MgCl2, and the mass ratio of the three was 10.00:0.25:1.00. CaCl2 and MgCl2 were the chloride precipitates contained in the formation water itself, not the precipitates formed by the reaction.
Fig. 3.
Fig. 3.
Scanning electron micrograph (a) and EDS image of precipitate (b).
The Ca2+ concentrations of initial formation water and the liquid taken out of the reactor after each experiment were tested by spectrum analyzer. The initial Ca2+ concentration of the formation water minus the Ca2+ concentration of the liquid taken after each experiment was the Ca2+ concentration reduction value of the formation water under the corresponding temperature and pressure difference conditions, and then the mass of the precipitate was calculated according to the relative molecular mass of CaCO3 (converted according to 1 L water sample, the results are shown in Table 2).
Table 2 Reaction results of different formation water samples under different pressure differences and temperatures.
| Formation water sample | Tempera- ture/°C | Pressure difference/MPa | Precipitate amount/mg |
|---|---|---|---|
| 1 | 20 | 8 | 310 |
| 20 | 10 | 330 | |
| 20 | 12 | 390 | |
| 20 | 16 | 510 | |
| 30 | 16 | 220 | |
| 50 | 16 | 150 | |
| 80 | 16 | 70 | |
| 2 | 20 | 8 | 170 |
| 20 | 10 | 200 | |
| 20 | 12 | 170 | |
| 20 | 16 | 240 | |
| 30 | 16 | 190 | |
| 50 | 16 | 100 | |
| 80 | 16 | 50 | |
| 3 | 20 | 8 | 300 |
| 20 | 10 | 470 | |
| 20 | 12 | 480 | |
| 20 | 16 | 570 | |
| 30 | 16 | 480 | |
| 50 | 16 | 240 | |
| 80 | 16 | 80 |
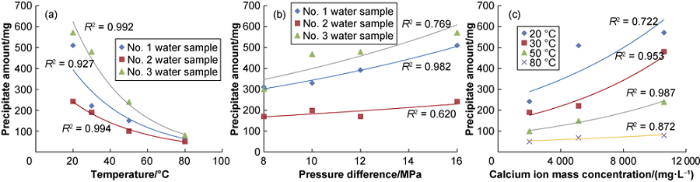
A graph was drawn according to the data in Tables 1 and 2 (Fig. 4). It can be seen that temperature, pressure difference, and mass concentration of calcium ions (scaling ions) have great influences on the generation of precipitates, and the temperature is inversely proportional to the amount of precipitate. The pressure difference and mass concentration of scaling ions are proportional to the amount of precipitate. The high values of correlation coefficients in the figure indicate that characterizing the trend of the experimental results in exponent form is accurate.
Fig. 4.
Fig. 4.
Relationship curves between temperature, pressure difference, calcium ion mass concentration and precipitate amount.
1.2.2. Displacement experiment
Displacement experiments of distilled water group and formation water group were carried out respectively. The core porosity and permeability test data before and after the displacement experiment are shown in Table 3. The core porosity change rate curves of distilled water group and formation water group before and after the experiment under different temperatures (displacement pressure difference is 6 MPa) and different pressure differences (temperature is 20 °C) are shown in Fig. 5. It can be seen from the figure that the porosity change rate is positively correlated with the pressure difference and temperature. Further comparison shows that the porosity increase of the core after the formation water displacement was always lower than that of core after the distilled water displacement. This is because the distilled water only corroded the core, while in the formation water group, the CO2-water interaction generated CaCO3 precipitate, which greatly affected the physical properties of the core during CO2 flooding.
Table 3 Core porosity and permeability tested before and after the experiment.
| Experiment No. | Displacement fluid | Temperature/ °C | Displacement pressure difference/MPa | Displacement pressure/ MPa | Calcium ion mass concentration/ (mg·L-1) | Permeability before experiment/ 10-3 μm2 | Permeability after experiment/ 10-3 μm2 | Permeability change rate/% | Porosity before experiment/ % | Porosity after experiment/ % | Porosity change rate/% |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Distilled water | 20 | 3 | 19 | 0 | 0.1219 | 0.1233 | 1.14848 | 8.50 | 8.62 | 1.41176 |
| 2 | 20 | 4 | 20 | 0.1222 | 0.1245 | 1.88216 | 8.61 | 8.81 | 2.32288 | ||
| 3 | 20 | 5 | 21 | 0.1266 | 0.1316 | 3.94944 | 8.83 | 9.18 | 3.96376 | ||
| 4 | 20 | 6 | 22 | 0.2664 | 0.2847 | 6.86936 | 7.90 | 8.55 | 8.22784 | ||
| 5 | 30 | 6 | 22 | 0.3585 | 0.3898 | 8.73082 | 9.01 | 10.31 | 14.42840 | ||
| 6 | 50 | 6 | 22 | 0.389 0 | 0.4392 | 12.90480 | 11.57 | 13.66 | 18.06390 | ||
| 7 | 80 | 6 | 22 | 0.4032 | 0.538 0 | 33.43250 | 11.68 | 15.45 | 32.27740 | ||
| 8 | Formation water | 20 | 3 | 19 | 10590 | 0.1294 | 0.1307 | 1.00463 | 8.05 | 8.10 | 0.62111 |
| 9 | 20 | 4 | 20 | 0.1299 | 0.1319 | 1.53964 | 8.41 | 8.49 | 0.95124 | ||
| 10 | 20 | 5 | 21 | 0.1316 | 0.1357 | 3.11550 | 8.50 | 8.59 | 1.05882 | ||
| 11 | 20 | 6 | 22 | 0.2225 | 0.235 0 | 5.61797 | 8.96 | 9.12 | 1.78571 | ||
| 12 | 30 | 6 | 22 | 0.3688 | 0.392 0 | 6.29067 | 9.82 | 10.22 | 4.07332 | ||
| 13 | 50 | 6 | 22 | 0.3337 | 0.3589 | 7.55169 | 8.52 | 9.33 | 9.50704 | ||
| 14 | 80 | 6 | 22 | 0.3614 | 0.4678 | 29.44100 | 11.48 | 14.87 | 29.52960 |
Note: The experimental cores are 25.24 mm in diameter, 75.44 mm in length, and 72.04 g in mass on average.
Fig. 5.
Fig. 5.
Core porosity change rates of distilled water group and formation water group displacement experiments under different temperatures and pressure differences.
2. Mathematical characterization method
It can be seen from Fig. 4 that the amount of inorganic salt precipitate has exponential relationships with temperature, pressure difference, and the mass concentration of scaling ions. In Fig. 5, the change in reservoir physical properties also has exponential relationships with temperature and pressure. Excel's data analysis tool was used to perform mathematical regression to first establish a equation:
Straighten the curve and take the logarithm of the two ends of the equation, then:
Perform linear regression analysis on lny and X to obtain a and b values. Set Y=lny, Y changes with the change of X, set:
Expand equation (5) and take the average of X, Y, XY, X2, Y2,$\overline{X}$,$\overline{Y}$,$\overline{XY}$,$\overline{{{X}^{2}}}$,$\overline{{{Y}^{2}}}$:
Q calculates the partial derivatives of a and b respectively, and sets the partial derivatives to zero to obtain the equations for solving a and b:
2.1. The mathematical characterization equation of precipitate amount
Regress the experimental data in Table 2 according to the above method to obtain the characterization equation:
The root mean square error of the mass concentration of scaling ions, temperature, and pressure difference are 9×10-5, 3×10-2, 5×10-2, respectively, indicating that the parameter accuracy is high, and their P values (assumed probability) are all less than 0.0001, so it can be considered that the confidence of the model reaches 99.99%.
2.2. Mathematical characterization equation of the degree of precipitate influence on reservoir physical properties
In the same way, the porosity change rate of the core in the distilled water displacement experiment in Table 3 was regressed to obtain the quantitative characterization equation of the porosity change rate, that is, the porosity change rate of the core under the effect of dissolution:
The porosity change rate of the core in the formation water displacement experiment in Table 3 was regressed to obtain the quantitative characterization equation of the porosity change rate, that is, the porosity change rate of the core under the combined effect of dissolution and precipitation:
Then the porosity change rate caused by precipitation is:
The porosity of the core after CO2 flooding is:
The Kozeny-Carman equation is used to describe the porosity-permeability relationship as follows[16]:
Substituting the experimental data in this study into the equation to get N=1, and substituting equation (12) into the above equation, the permeability is obtained as:
3. The influence of precipitation on oil recovery
3.1. Reservoir numerical simulation model considering inorganic salt precipitation
A reservoir geological model was built based on the reservoir properties of the research block; the fluid properties of the research block were taken to establish a typical numerical model, and equation (8) obtained by regression was used to fit the precipitate amount; equations (12) and (14) were used to fit porosity and permeability respectively and then revised, and the reactant H2O coefficient and chemical reaction speed were worked out.
The reservoir in the research block has an average porosity of 10.01% and an atmospheric permeability of 0.3×10-3 μm2, representing tight oil reservoir with extremely low asphaltene content. The formation water has high Ca2+ content of up to 10590 mg/L. The reservoir has a burial depth of 2700-2900 m, a formation temperature of 80 °C, and a formation pressure of 21 MPa. The reservoir is developed by waterflooding and vertical well diamond reverse nine-point well pattern. It has a recovery rate of 15% and the comprehensive water cut of 53% now. The E300 module of Eclipse numerical simulation software was used to simulate the influence of sediments generated by the interaction of CO2 and formation water on the oilfield recovery under continuous CO2 flooding.
In the E300 module of the Eclipse numerical simulation software, the solid component was added to the fluid component through a chemical reaction. At this time, the saturations of oil, gas, and water changed, and the fluid saturation equation included the solid saturation:
The solid phase in the fluid can precipitate or be produced with the fluid from the production well. The precipitate will reduce the permeability of the reservoir and has adverse effect on the physical properties of the reservoir. Assuming that the solid phase generated in the fluid is all absorbed to the reservoir but not produced with the fluid, the precipitate amount can be calculated equivalently. Equations (8), (12), and (14) were used to get the corrected reaction rate coefficient and reactant H2O coefficient, then the saturation of solid precipitate in (15) was multiplied with the pore volume to get the amount of precipitate. Some parameters of the model are shown in Table 4.
Table 4 Model parameters
| Parameters | Value | Parameters | Value |
|---|---|---|---|
| Oil viscosity | 1.81 mPa·s | Reservoir temperature | 80 °C |
| Permeability | 0.2×10-3 μm2 | Rock density | 2500 kg/m3 |
| Porosity | 10% | Solution gas-oil ratio | 43 m3/m3 |
| Initial water saturation | 53% | Initial oil saturation | 60% |
| Reactant CO2 coefficient | 1 | Reaction rate coefficient | 5×10-6 |
| Reactant H2O coefficient | 1 280 |
3.2. Analysis of the influence of inorganic salt precipitation on oil recovery
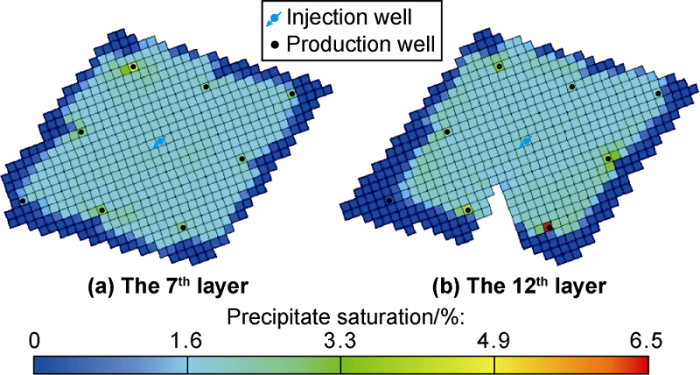
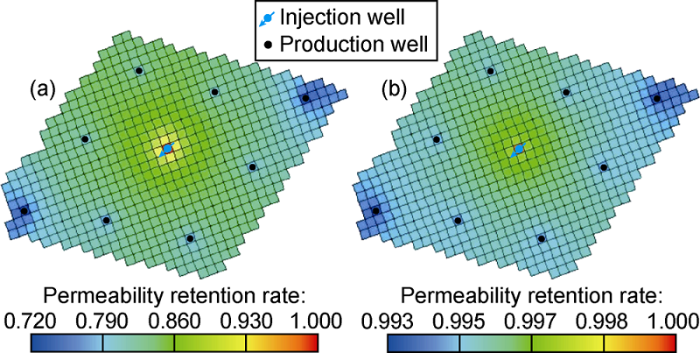
Fig. 6 shows the distribution of sediments in the reservoir after 20 years of CO2 flooding. It can be seen that the precipitate is relatively evenly distributed in the entire area, and a large amount of precipitate deposits near the bottom of the well. This is due to the large pressure difference near the production well. Fig. 7 shows the distribution of porosity retention rate (ratio of porosity to original porosity) and permeability retention rate (ratio of permeability to original permeability) after 20 years of CO2 flooding. It can be seen that after 20 years of CO2 flooding, precipitate is distributed in the entire well group. The sedimentation and migration of the precipitate cause plugging of pores and thus reduction of reservoir porosity and permeability.
Fig. 6.
Fig. 6.
Simulation results of inorganic salt precipitate distribution after 20 years of CO2 flooding.
Fig. 7.
Fig. 7.
Distribution of permeability retention rate and porosity retention rate after 20 years of CO2 flooding.
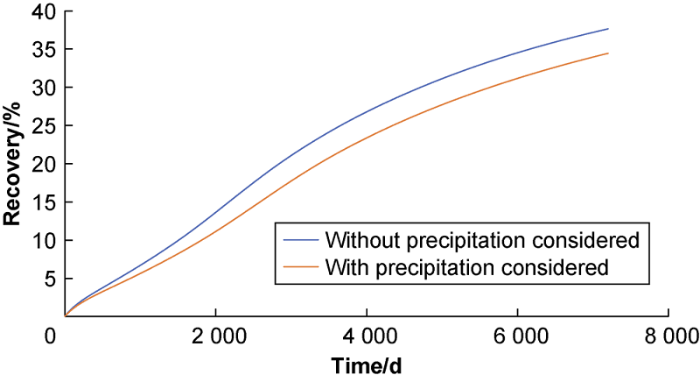
Fig. 8 shows the oil recovery curves over 20 years of CO2 flooding of the oilfield predicted by the model with and without the influence of precipitation considered. It can be seen from the figure that and precipitation begins to affect the recovery of the oilfield 5 years into the flooding; as the development goes on, more and more precipitate is produced, blocking the pore throats and making the recovery rate drop. After 20 years of production, the ultimate recovery rate without considering the effect of precipitation is 37.64%, while the ultimate recovery rate considering the effects of precipitation is 33.45%.
Fig. 8.
Fig. 8.
Oil recovery factor after 20 years of CO2 flooding development with and without precipitation considered.
4. Conclusions
The inorganic salt precipitate generated during the CO2-formation water reaction is mainly CaCO3, and the pressure difference and mass concentration of scale- forming ions are proportional to the amount of precipitate, and the temperature is inversely proportional to the amount of precipitate. The change rate of core porosity before and after CO2 flooding is positively correlated with temperature and displacement pressure difference. Due to the effect of precipitation, the increase in porosity of formation water group cores is always lower than that of distilled water group cores.
With the continuous injection of CO2, the most precipitates will eventually be produced near the production well. Compared with water flooding development, CO2 flooding development has significantly improved the effect, and the recovery rate has increased from 15% to 33.45%-37.64%. Due to the extensive deposition of precipitation in the well group, the effect of oilfield development has deteriorated. The block recovery rate is 33.45% when precipitation is considered, and 37.64% when precipitation is not considered.
Nomenclature
a, b—coefficients;
i—No. of influencing factors;
Is—saturation index;
K—function of system temperature and ionic strength;
K0—initial core permeability, 10-3 μm2;
Kt—core permeability after CO2 flooding, 10-3 μm2;
M—scaling ion mass concentration, mg/L;
N—relation index;
n—number of influencing factors;
PAlk—negative logarithm of total alkalinity concentration;
PCa—negative logarithm of the concentration of Ca2+;
pH—pH value of the water system;
pHs—pH value of the solution saturated with CaCO3;
Q(a, b)—function;
Sg—gas saturation, %;
So—oil saturation, %;
Ss—solid saturation, %;
Sw—water saturation, %;
t—temperature, °C;
X—influencing factors;
y—precipitate amount, mg;
Δp—pressure difference, MPa;
ϕ0—initial core porosity, %;
Δϕ1—change rate of core porosity under dissolution, %;
Δϕ2—change rate of core porosity under combined effect of dissolution and precipitation, %;
Δϕ3—porosity change rate of the core under the effect of precipitation, %;
ϕt—porosity of the core after CO2 flooding, %.
Reference
Simulation for the dissolution mechanism of Cambrian carbonate rocks in Tarim Basin, NW China
Technologies and practice of CO2 flooding and sequestration in China
Experimental study on the growth behavior of supercritical CO2-induced fractures in a layered tight sandstone formation
Experimental study on fracture initiation and propagation in shale using supercritical carbon dioxide fracturing
Impacts of CO2-brine-rock interaction on sealing efficiency of sand caprock: A case study of Shihezi Formation in Ordos Basin
An improved method for predicting CO2 minimum miscibility pressure based on artificial neural network
Analysis and prediction of scaling mechanism of the CaCO3 from oilfield injection brines
Review of the CO2-brine-rock interaction in reservoir
An experimental study on water-rock interaction during water flooding in formations satuated with CO2
The dissolution effects of CO2-brine systems on the permeability of U.K. and North Sea calcareous sandstones
The influence of CO2-water-calcite interactions on surface texture and permeability of the calcite
Analytical solution to evaluate salt precipitation during CO2 injection in saline aquifers
Numerical modeling of formation damage by two-phase particulate transport processes during CO2 injection in deep heterogeneous porous media
Temperature effect on carbonic acid balance in brine
Equilibrium chemistry of the CaCO3-CO2-H2O system and discussions
Modeling CO2 storage in aquifers with a fully-coupled geochemical EOS compositional simulator