Introduction
Mokhtari et al.[3] studied Xanthan-borate system and the effect of pH, polymer concentration, and shear rate, on the gelation behavior. They observed that pH and polymer concentration accelerate the gelation process, but, shear rate does not have an impressive effect. They, also, presented an empirical model to simulate the behavior of Xanthan-borate system.
Guar is cross-linked by ions such as titanate, zirconate, aluminate and borate[4]. Borate ion is the most commonly used ion for cross-linking[5]. There are numerous parameters such as temperature, pressure, and shear stress that affect cross- linking process. Increasing the temperature reduces inter- chain contacts exponentially and weakens the base polymer. In order to compensate this effect, pH must be increased, which leads to higher concentration of borate ion. Hence, polymer should be used at higher concentrations to achieve the appropriate final viscosity. Due to re-healing process, cross-linked bond is broken and reformed again, which means that the shear stress does not damage borate-Guar cross-linked gel permanently. Time required to re-cross-link through polymer chains is less than 1 ms, due to the rapid exchange equilibrium of mono-borate ion and borate acid[6]. Viscosity of Guar cross-linked gel is reduced at high pressures, but this reduction is also reversible[7].
Polyacrylamides are the most common polymers used in operations such as water shutoff. These polymers are inexpensive and can be cross-linked by metallic and organic cross-linkers. Moradi-Araghi et al.[8,9,10] reported cross-linking of polyacrylamide to different extents of hydrolysis and molecular weights with metallic and organic cross-linkers for diversion applications. Upendra et al.[11] cross-linked HPAM with hexamine and hydroquinone cross linkers to devise a gel system. The developed gel would be injected to block the highly permeable or watered-out sections of reservoirs. They showed that the viscosity of PHPA-hexamine-hydroquinone gel system increases by increasing the ageing time, polymer concentration, temperature, and cross-linker concentration. Also they found that PHPA-hexamine-hydroquinone gel system is affected by salinity and pH.
Graphitic carbon in nanoscale structure is called carbon nanotube (CNT). Carbon nanotube is composed of graphene sheets rolled to make tubes. Multiple concentric graphene cylinders are used to make Multi-walled carbon nanotubes (MWNTs). At the time, the most commercially accessible form of CNTs are MWNTs[12]. The rheological behavior of CNTs suspensions has been investigated by Ma et al.[13] and Nobile[14]. Kathryn et al.[15] have experimentally investigated rheological behavior of MWNT suspensions in a Newtonian epoxy medium. They found that MWCNs can improve the rheological behavior of the suspensions.
The aim of this work is to determine the effects of cations on rheology and stability of different cross-linked Guar, Xanthan and HPAM gels at different pressures. Also, a new approach was presented to enahnce rheology and stability of polymers in confronting with cations by using nanoparticles.
1. Methodology
1.1. Dynamic filtration setup
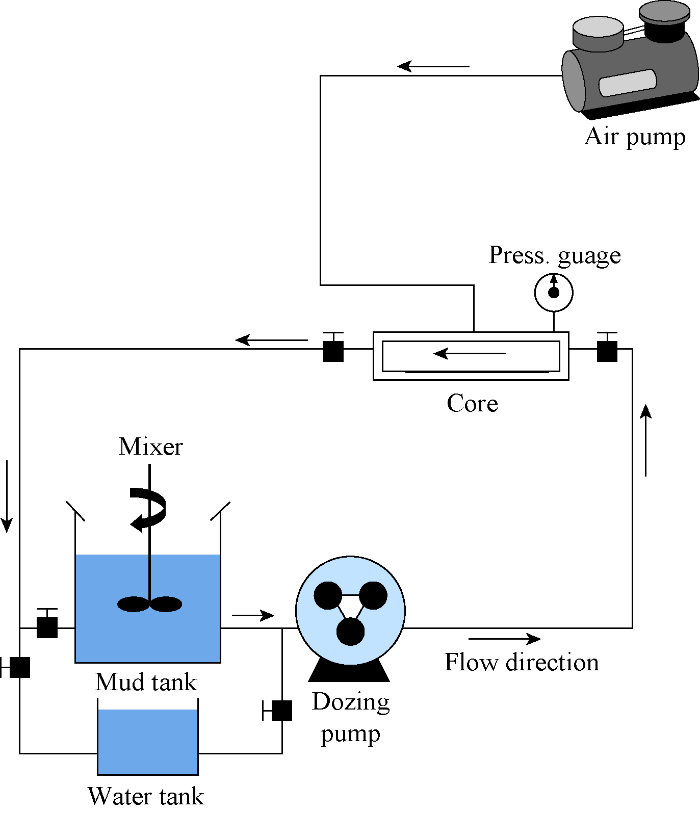
In order to study the effect of differential pressure on the performance of cross-linked gel, a dynamic filtration setup consisting of a flooding core holder, a mud tank, a static tank, a dozing pump, an air compressor, and a mixer was designed (Fig. 1). The core holder cell was made of steel and has two input and one output flow paths. The first input is connected to the dosing pump to adjust the flow rate and the second one is connected to the air pump to seal the core body.
Fig. 1.
Fig. 1.
Schematic diagram of the dynamic filtration setup.
The cylindrical core sample, was a tight carbonate rock which taken from Asmari formation outcrop in Ahvaz oil filed of Iran and composed of two half-cylinders placed next to each other with a 1 mm gap in the middle to mimic the fracture in the formation during drilling. Diameter of the cylinder was 3.8 cm and its length was 8 cm (Fig. 2).
Fig. 2.
Fig. 2.
The cylindrical core used in the filtration experiment.
1.2. Materials
Different polymers and cross-linkers used in this study were provided by National Iranian Drilling Company, and they are being used in well drilling operations. Xanthan, Guar, and PHPA polymers were used in our experiment. Borax was employed as the cross-linker for Xanthan and Guar, and APTES was chosen as the cross-linker for PHPA. To determine the effect of salinity on the cross-linked gel, NaCl and CaCl2 solutions were used in different concentrations. Additionally, MWCNTs were purchased from Carbon Nano Technology Pishroo Co. and added to PHPA cross-linked gel combination to improve the performance of the gel. MWCNTs were dispersed in water; to enhance the stability of nanotubes in water, nitric acid was used in our procedure. NaOH was also used as the pH buffer to accelerate the gelation process.
Rheological parameters were measured according to API 13D, by FANN Viscometer-Model 35. Also the Fourier-transform Infrared Spectroscopy (FTIR) test was applied to identify different types of materials.
1.3. Nano fluid preparation and functionalization
The following procedure was applied to prepare MWCNT[16][12]: (1) Combination of HNO3 (C = 69% v/v) and MWCNTs at the ratio of 0.1 g nanoparticle for 20 mL nitric acid; (2) Reflux them at 140 °C for 3 hours; (3) Separating MWCNTs from the acid by centrifuging; (4) Separating MWCNTs from the acid by centrifuging; (5) Separation of MWCNTs from the water; (6) Repeating steps 4 to 5 for three times.
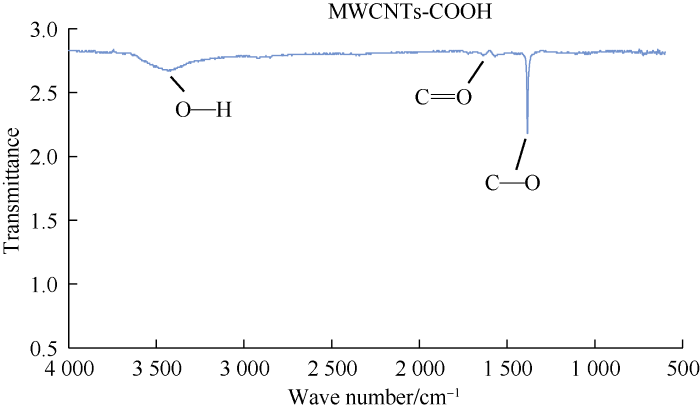
After functionalization and connection of polar functional groups (-COOH) to nanoparticles, carbon nanotubes dispersion were more favorably in the water. Fig. 3 shows the FTIR test result of the functionalized MWCNTs. As shown in this figure, functional groups (-COOH) are connected to nanoparticles which approves the presence of functionalized MWCNTs in the final fluid.
Fig. 3.
Fig. 3.
FTIR result of functionalized MWCNTs.
1.4. Cross-linked Gel preparation
To prepare different gels, first various concentrations of NaCl/CaCl2 were solved in 350 mL water. For each type of gel, specific polymer was added to brine and mixed for 10 min at 100 s-1. Then the appropriate cross-linking agent was added to the combination and mixed for another 5 min at 100 s-1. At the last step, 3.5 mL NaOH (0.1 M) was added to the cross-linked gel and mixed for 3 min at the same speed. To prepare the improved PHPA, 1% of dry functionalized MWCNTs were also added to the brine at the first stage.
Different samples of cross-linked gel were prepared at various salinities as shown in Table 1. For any gel combination, viscosity was measured to study the rheological behavior of the gel at low and high salinities. Gelation behavior of cross-linked polymers was determined by steady shear viscometry method[17]. In this method, apparent viscosity (AV) of fluid is plotted versus time, at steady shear rate at 0.05 s-1.
Table 1 Cross-linked gel combination for various tests.
| Polymer (Mass fraction) | Cross-linker (Mass fraction) | CaCl2/ 10-6 | NaCl/ 10-6 |
|---|---|---|---|
| Xanthan (2%) | Borax (1%) | 0 | 0 |
| Xanthan (2%) | Borax (1%) | 2 000 | 0 |
| Xanthan (2%) | Borax (1%) | 4 000 | 0 |
| Xanthan (2%) | Borax (1%) | 6 000 | 0 |
| Xanthan (2%) | Borax (1%) | 8 000 | 0 |
| Xanthan (2%) | Borax (1%) | 10 000 | 0 |
| Xanthan (2%) | Borax (1%) | 0 | 2 000 |
| Xanthan (2%) | Borax (1%) | 0 | 4 000 |
| Xanthan (2%) | Borax (1%) | 0 | 6 000 |
| Xanthan (2%) | Borax (1%) | 0 | 8 000 |
| Xanthan (2%) | Borax (1%) | 0 | 10 000 |
| Guar (2%) | Borax (1%) | 0 | 0 |
| Guar (2%) | Borax (1%) | 2 000 | 0 |
| Guar (2%) | Borax (1%) | 4 000 | 0 |
| Guar (2%) | Borax (1%) | 6 000 | 0 |
| Guar (2%) | Borax (1%) | 8 000 | 0 |
| Guar (2%) | Borax (1%) | 10 000 | 0 |
| Guar (2%) | Borax (1%) | 0 | 2 000 |
| Guar (2%) | Borax (1%) | 0 | 4 000 |
| Guar (2%) | Borax (1%) | 0 | 6 000 |
| Guar (2%) | Borax (1%) | 0 | 8 000 |
| Guar (2%) | Borax (1%) | 0 | 10 000 |
| HPAM(10%) | APTES(3%) | 0 | 0 |
| HPAM(10%) | APTES(3%) | 2 000 | 0 |
| HPAM(10%) | APTES(3%) | 4 000 | 0 |
| HPAM(10%) | APTES(3%) | 6 000 | 0 |
| HPAM(10%) | APTES(3%) | 8 000 | 0 |
| HPAM(10%) | APTES(3%) | 10 000 | 0 |
| HPAM(10%) | APTES(3% ) | 0 | 2 000 |
| HPAM(10%) | APTES(3%) | 0 | 4 000 |
| HPAM(10%) | APTES(3%) | 0 | 6 000 |
| HPAM(10%) | APTES(3%) | 0 | 8 000 |
| HPAM(10%) | APTES(3%) | 0 | 10 000 |
| HPAM*(10%) | APTES(3%) | 0 | 0 |
| HPAM*(10%) | APTES(3%) | 2 000 | 0 |
| HPAM*(10%) | APTES(3%) | 4 000 | 0 |
| HPAM*(10%) | APTES(3%) | 6 000 | 0 |
| HPAM*(10%) | APTES(3%) | 8 000 | 0 |
| HPAM*(10%) | APTES(3%) | 10 000 | 0 |
| HPAM*(10%) | APTES(3%) | 0 | 2 000 |
| HPAM*(10%) | APTES(3%) | 0 | 4 000 |
| HPAM*(10%) | APTES(3%) | 0 | 6 000 |
| HPAM*(10%) | APTES(3%) | 0 | 8 000 |
| HPAM*(10%) | APTES(3%) | 0 | 10 000 |
Note: *HPAM in presence of nanoparticle
Addition of salt to water decreases the overall gel viscosity as shown by Mahmood et al.[18]. Hence, in all tests, based on Maryann et al. [19] study, an upper limit of salt concentration was fixed at 10 000×10-6, to prevent the reduction in the polymer solvency at very high salinities which affects the resultant cross-linked gels properties
2. Results and discussion
2.1. Effect of cation type and concentration on the gelation process
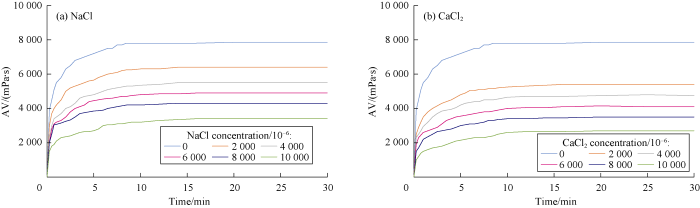
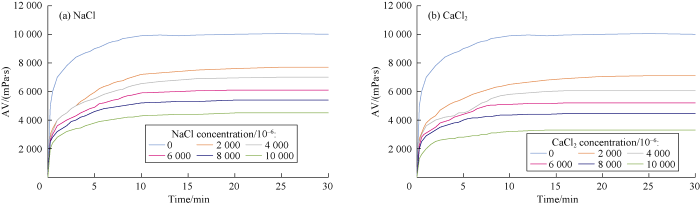
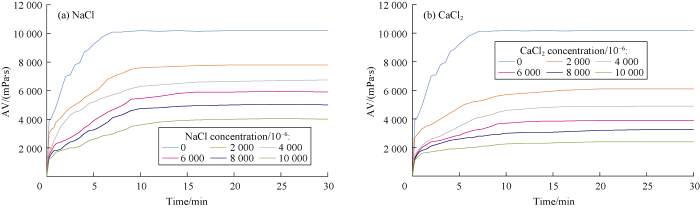
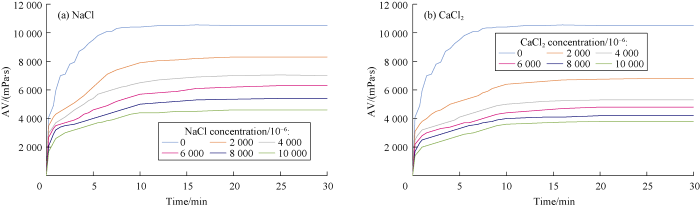
AV values of gel combination versus time were measured at constant 30 °C temperature for different concentrations of NaCl and CaCl2 to study the effects of salt concentration on the rheological behavior of polymers. Cations react with the anionic and natural polymers and cause precipitation in the solution, which consequently reduces the viscosity. The higher the concentration of cations, the more severe is the reduction in viscosity. This effect was measured for different cross- linked polymers at different concentration of NaCl and CaCl2 during 30 minutes as shown in Figs. 4 through 7.
Fig. 4.
Fig. 4.
Effects of NaCl and CaCl2 concentrations on Borate-Xanthan gelation behavior.
Fig. 5.
Fig. 5.
Effects of NaCl and CaCl2 concentrations on Borate-Guar gelation behavior.
Fig. 6.
Fig. 6.
Effects of NaCl and CaCl2 concentrations on APTES-HPAM gelation behavior.
Fig. 7.
Fig. 7.
Effects of NaCl and CaCl2 concentrations on APTES-HPAM-MWCNT gelation behavior.
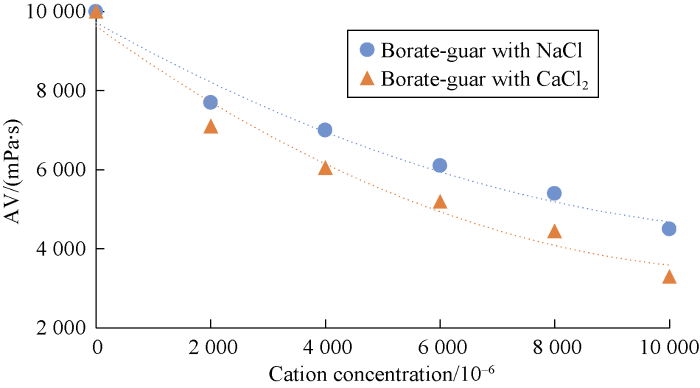
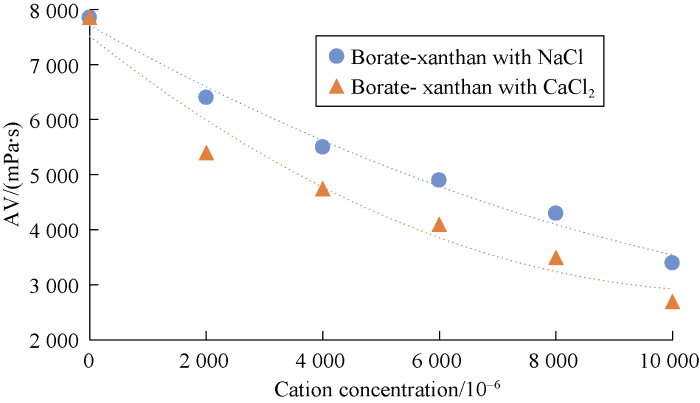
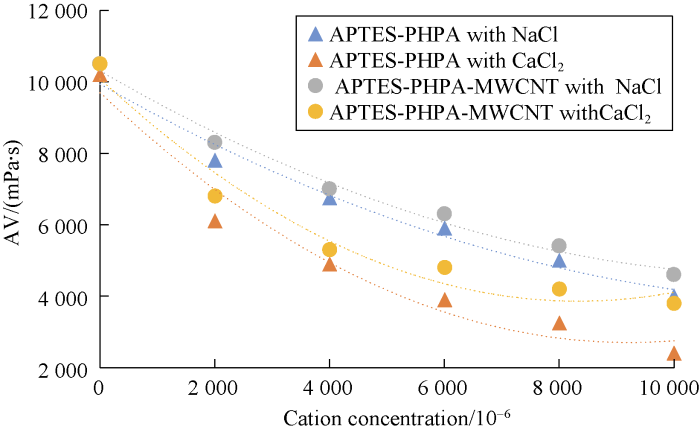
Comparing behaviors of NaCl and CaCl2 shows that divalent cations cause more viscosity reduction in comparison to monovalent cations. The relationship between cation concentration and viscosity of cross-linked gels after 30 minutes are presented in Figs. 8 through 10 for different cross-linked polymers. Also, the rheological behavior was compared for HPAM cross-linked polymer in presence and absence of MWCNTs. Our tests showed that the reduction in viscosity was lower in the new developed HPAM/MWCNT gel. Also cross-linked gel of Guar showed higher viscosity in comparison to Xanthan, which means that the Guar gel is stronger than Xanthan. Viscosity reduction models based on these curves were developed and listed in Table 2 based on Figs. 8 through 10.
Fig. 8.
Fig. 8.
Effects of cation concentrations on viscosity of borate- Guar after 30 minutes.
Fig. 9.
Fig. 9.
Effects of cation concentrations on viscosity of borate-Xanthan after 30 minutes.
Fig. 10.
Fig. 10.
Effects of cation concentrations on viscosity of APTES- HPAM and APTES-HPAM-MWCNTs after 30 minutes.
Table 2 Viscosity reduction models.
| NUM | Equation | R2 | Related curve description |
|---|---|---|---|
| 1 | Y=9.721×103-0.817X+3.125×10-5X2 | 0.976 4 | Effects of cation concentrations on viscosity of borate-Guar with NaCl |
| 2 | Y=9.630×103-1.048X+4.442×10-5X2 | 0.970 8 | Effects of cation concentrations on viscosity of borate-Guar with CaCl2 |
| 3 | Y=7.709×103-0.593X+1.763×10-5X2 | 0.988 2 | Effects of cation concentrations on viscosity of borate-Xanthan with NaCl |
| 4 | Y=7.512×103-0.836 X+3.772×10-5X2 | 0.960 3 | Effects of cation concentrations on viscosity of borate-Xanthan with CaCl2 |
| 5 | Y=9.936×103-0.914X+3.393×10-5X2 | 0.983 5 | Effects of cation concentrations on viscosity of APTES-HPAM with NaCl |
| 6 | Y=9.691×103-1.517X+8.237×10-5X2 | 0.962 4 | Effects of cation concentrations on viscosity of APTES-HPAM with CaCl2 |
| 7 | Y=1.031×103-0.940X+3.839×10-5X2 | 0.989 0 | Effects of cation concentrations on viscosity of APTES-HPAM-MWCNTs with NaCl |
| 8 | Y=1.008×103-1.494X+8.973×10-5X2 | 0.965 3 | Effects of cation concentrations on viscosity of APTES-HPAM-MWCNTs with CaCl2 |
2.2. Effect of MWCNTs on cross-linked gel resistance at different salinities
As can be observed from Figs. 8 through 10, presence of cation causes reduction in cross-linked gel viscosity. By comparing curves in Fig. 10, it can be concluded that the viscosity of HPAM cross-linked gel in presence of MWCNTs is higher, which shows the positive effect of nanoparticle on the stability of the cross-linked gel against the cation. MWCNTs protect the polymer from cation reactions. Hence, lower reduction in viscosity was observed. For instance, by adding the nanotubes to the APTES-HPAM in presence of NaCl, there would be an average of 10% improvement in viscosity. After functionalization of MWCNTs, COOH group provides a negative charge on nanoparticle’s body which provides an anionic behavior. Therefore, no bond is formed between functionalized nanoparticles and anionic HPAM polymers. Due to high specific area of MWCNTs (200 m2/g), they surround cations and remove them from polymers which reduces the chance of reaction between cations and polymer chains. This process is somewhat similar to encapsulating cations. Additionally, this effect is more impressive at severe conditions such as high cation concentration. The effect of MWCNTs on the gelation process is more dominant in the presence of CaCl2, which shows the ability of MWCNTs to control the higher precipitation of HPAM in presence of divalent cations.
2.3. Effect of cations on the cross-linked gel stability at different pressures
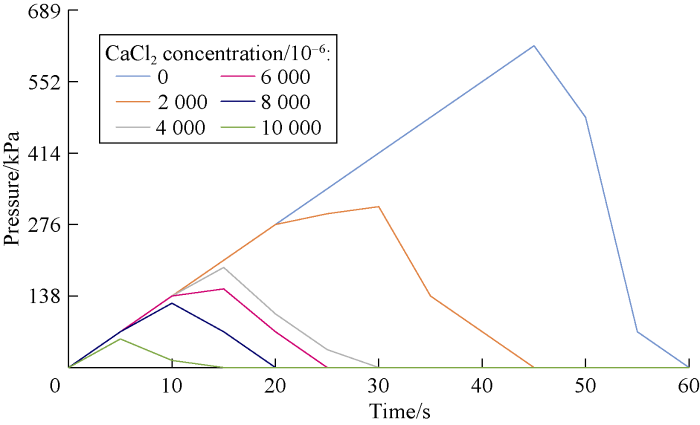
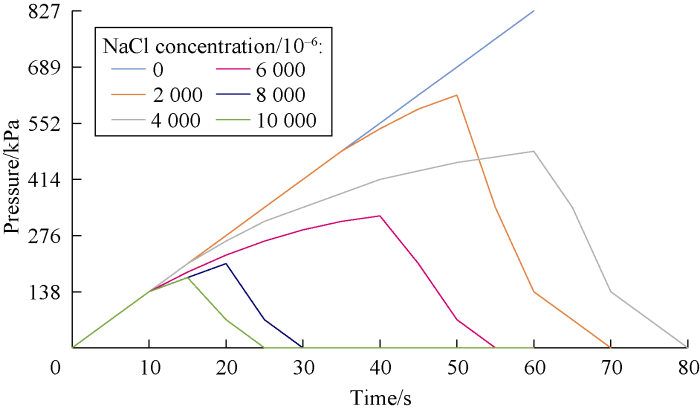
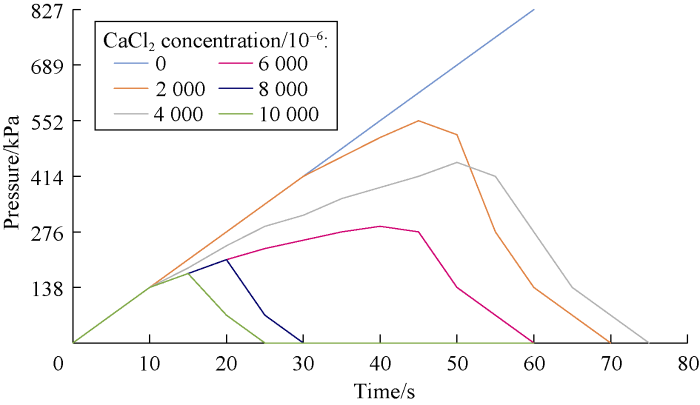
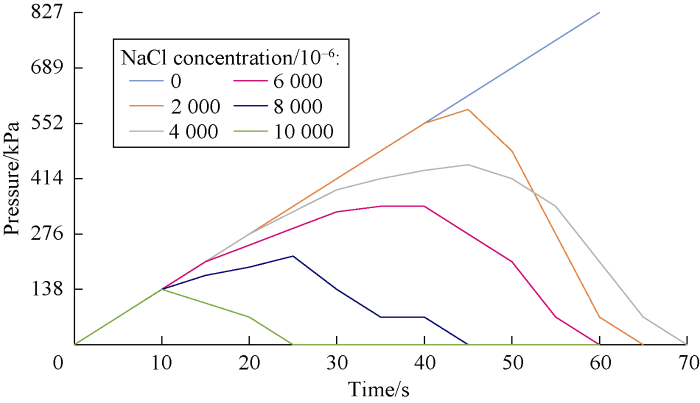
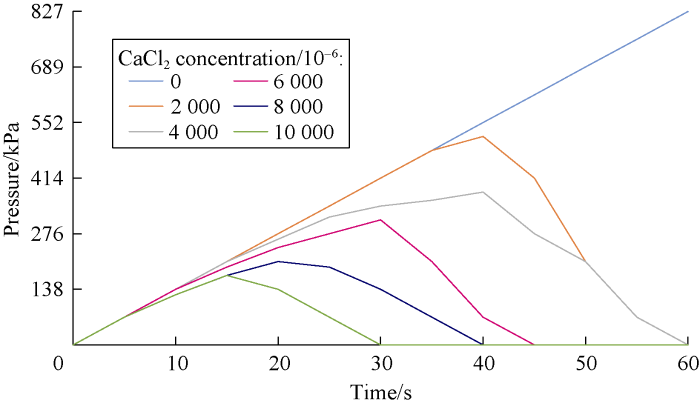
An important part of our study was to investigate the dynamic stability of cross-linked gels in the fractured formation by the designed dynamic filtration setup. Cross-linked gels were injected to the fracture of the core by the pump pressure. Then, the pump was turned off and input and output valves of core holder cell were closed in order to keep the pressure at 689 kPa in the cell. After about four hours, the gel became rigid. Then the valves were opened and pump was turned on to inject the drilling mud (prepared by bentonite and freshwater) to the fracture filled with the gel. Stability and resistance of the gel were analyzed by monitoring the pressure of the gauge at the inlet of the cell. If the pressure rose, this means that the gel was stable and was not break down under the pressure. However, if the pressure dropped, it means that there was a leakage or a breakdown in the cross-linked gel in the fracture. Figs. 11 through 18 show pressure profiles versus time for different gels to study the stability.
Fig. 11.
Fig. 11.
Effect of salinity on Xanthan cross-linked gel stability at different concentrations of NaCl.
Fig. 12.
Fig. 12.
Effect of salinity on Xanthan cross-linked gel stability at different concentrations of CaCl2.
Fig. 13.
Fig. 13.
Effect of salinity on Guar cross-linked gel stability at different concentrations of NaCl.
Fig. 14.
Fig. 14.
Effect of salinity on Guar cross-linked gel stability at different concentrations of CaCl2.
Fig. 15.
Fig. 15.
Effect of salinity on HPAM cross-linked gel stability at different concentrations of NaCl.
Fig. 16.
Fig. 16.
Effect of salinity on HPAM cross-linked gel stability at different concentrations of CaCl2.
Fig. 17.
Fig. 17.
Effect of salinity on HPAM-MWCNTs cross-linked gel stability at different concentrations of NaCl.
Fig. 18.
Fig. 18.
Effect of salinity on HPAM-MWCNTs cross-linked gel stability at different concentrations of CaCl2.
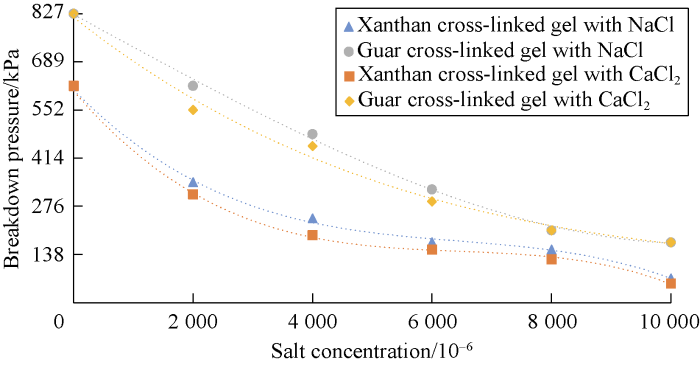
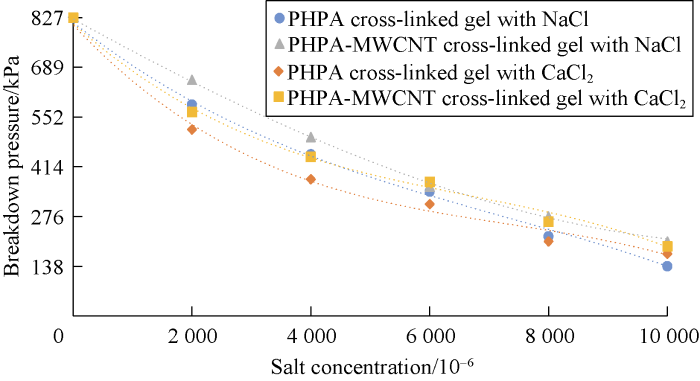
Effects of salt concentration on breakdown pressure for different gel systems are shown in Figs. 19 and 20. These two figures can be used to compare the performance of different gels at various pressures. Also the benefits of the developed gel can also be analyzed.
According to Figs. 19 and 20, cation causes reduction in the gel stability and decreases the maximum pressure that the cross-linked gel can tolerate. This reduction is more at the higher salt concentrations. Also divalent cation causes more reduction in cross-linked gel stability compared to the monovalent one.
Fig. 19.
Fig. 19.
Breakdown pressure for Guar cross-linked gel and Xanthan cross-linked gel at different salinity.
Fig. 20.
Fig. 20.
Breakdown pressure for HPAM cross-linked gel and HPAM-MWCNTs cross-linked gel at different salinity.
According to Figs. 11 through 14, the effect of salinity on gel stability of Guar was somehow the same as Xanthan. The difference was in the stability of gels in cation free cases. Xanthan cross-linked gel was able to tolerate maximum pres-sure of 621 kPa and in this pressure the gel was broken down. However, this parameter for Guar cross-linked gel was more than 827 kPa.
According to Fig. 20, the stability of cross-linked gels ordinary reduces by increasing salt concentration, but in the case of using MWCNTs due to the encapsulating effect around molecules, breakdown pressure is promoted to some extent. NaCl is a better additive in comparison to CaCl2, as the reduction in breakdown pressure was lower in the final gel at different salinities. In the case of using MWCNTs in the cross-linked gel, the pressure stability of HPAM cross-linked gel with NaCl increased for about 12% by using HPAM conjunct with MWCNTs. Similar behavior was observed for HPAM-MWCNTs cross-linked gels with CaCl2. In this case the average pressure stability improvement was about 17%. Technically, based on our tests, HPAM-MWCNT is a good choice due to better performance and higher breakdown pressure. However, cost evaluation analysis is required to select the best gel for the field application.
3. Conclusions
The presence of cations in cross-linked gel system will reduce the viscosity of gel, the higher the cation concentration is, the lower the viscosity will be. The bivalent cation has a greater viscosity reduction effect on gel than monovalent cation. The stability of cross-linked gels is worse with cations, this situation becomes more serious under higher salinity. Guar cross-linked gel exhibited higher viscosity and gel stability in comparison to Xanthan cross-linked gel at the same concentration and presence and the same cross-linker agent.
With addition of MWCNT in HPAM gel, and through crosslinking by using APTES, MWCNTs showed appropriate effect on the cross-linked gel stability in presence of different cations by controlling the negative effect of cations on the viscosity which increases the HPAM cross-linked gel stability at different operational conditions. Hence, the new developed HPAM gel can be considered as an appropriate option to be applied in drilling operations.
Reference
Lost circulation control: Evolving techniques and srategies to reduce downhole mud losses
Unique crosslinking pill in tandem with fracture prediction model cures circulation losses in deepwater Gulf of Mexico
Laboratory investigation on gelation behavior of xanthan crosslinked with borate intended to combat lost circulation
A review of fracturing fluid systems used for hydraulic fracturing of oil and gas wells
Reservoir stimulation
Chemistry and rheology of borate-crosslinked fluids at temperatures to 300 ℉
Influence of pressure on boron cross-linked polymer gels
A review of thermally stable gels for fluid diversion in petroleum production
Hydrolysis and precipitation of polyacrylamides in hard brines at elevated temperatures
Stability of polyacrylamides in hard brines at elevated temperatures
Rheological investigation of partially hydrolyzed polyacrylamide-hexamine-hydroquinone gels
A review of the microstructure and rheology of carbon nanotube suspensions
Polymer-carbon nanotube composites: Preparation, properties and applications
The rheology of multiwalled carbon nanotube and carbon black suspensions
Evaluation of mild acid oxidation treatments for MWCNT functionalization
Rheological screening of low-molecular-weight polyacrylamide/chromium(III) acetate water shutoff gels
Effect of salinity on the viscosity of water based drilling fluids at elevated pressures and temperatures
Effect of salt on polymer solvency: Implications for dispersion stability