Introduction
Globally, carbonate reservoirs are widely distributed in Jurassic, Cretaceous, and Neogene in North America, the Middle East, and Central Asia, etc[1]. The main components of carbonate rock are calcite and dolomite, which have poor physical and chemical stability. After deposition, they often undergo transformations by various chemical/physical actions, resulting in changes in the specific surface, porosity, permeability, wettability and so on. The waterflooding recovery of this kind of reservoir is also affected by various activities of water-soluble ions in rock, water and crude oil to some extent.
Ion-matched waterflooding (or low-salinity water, smart waterflooding) improves oil recovery by adjusting the total salinity and ion composition of injected water, and has achieved good results in both laboratory and field tests. Compared with conventional water injection, the oil displacement efficiency of the ion-matched waterflooding can increase by up to 30% in the laboratory experiment, and the residual oil saturation can reduce by 2%-50% in field tests[5]. However,the application effects of ion-matched waterflooding in oilfields vary widely. By using ion-matched waterflooding, the water content of Burgan Oilfield in Kuwait reduced by 5%; the residual oil saturation of Upper Jurassic reservoirs in Saudi Arabia decreased by 6%; the Clair Ridge and Ekofisk oilfields in the north sea of the UK had remarkable effects, while the Valhall oilfield had no obvious effects[5]. The mechanisms of ion-matched waterflooding for EOR are complex, and the main control mechanism is controversial at present[6] and cannot effectively guide the field application. So the application effects differ widely in fields with low controllability.
Currently, the main application of ion-matched water is to dilute certain water or change the concentration of potential determining ion (PDI). PDI is closely related to oil recovery, mainly including Ca2+, Mg2+ and SO42-[7,8]. Currently, it is considered that multi-ion exchange, dissolution and other effects improve the wettability of rock, and the effect of key ions enhances oil-water interface performance, thereby improving recovery. However, the mechanism of efficient ion exchange in ion-matched water, the selection of optimal total salinity range and optimal PDI concentration are still unclear or contradictory[9,10,11].
In view of this, based on systematically reviewing the research results of ion-matched water, this study examined the diversity and synergy of the mechanism. In view of the problems and contradictions in using salinity to characterize the ionic behavior in aqueous solution, ionic strength (IS) was suggested to characterize the behavior differences of univalent and divalent ions to find out the relationship between IS, effective concentration and mechanisms, and evaluate the performance of ion-matched water. Based on in-depth analysis of the main waterflooding contradictions in the Mishrif Formation grain limestone reservoir of Halfaya oilfield in the Middle East, relative permeability, X-ray diffraction (XRD), and oil-water interfacial tension (IFT) tests were carried out to study the effect of ion behavior on enhanced oil recovery mechanism under the reservoir conditions. At the same time, core-flooding experiments were conducted to study the reasonable range of total salinity and IS of the injected water comprehensively, and select the ion-matched water type suitable for the reservoir combined with injection water evaluation method.
1. EOR Mechanisms of ion-matched waterflooding
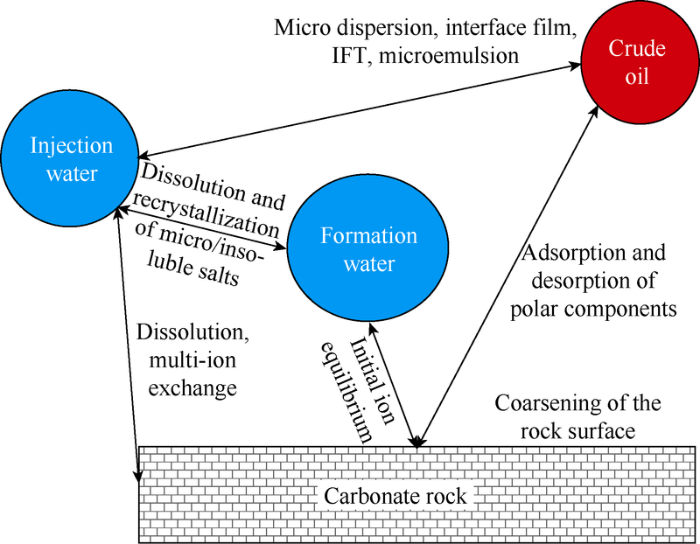
The mechanisms of ion-matched water flooding can be classified according to the interfaces (Fig. 1). To emphasize the interaction between the mechanisms, they can also be classified according to direct or indirect influences on oil displacement efficiency (Table 1). Among them, the secondary mechanism I (exchange of multiple kinds of ions) will work firstly (occurring in less than 15 min in the experiment) and then influence the other mechanisms[12]. The mechanisms are diverse and synergetic.
Fig. 1.
Schematic diagram of interface behavior after ion- matched water is injected into reservoirs.
Table 1 Classification of mechanisms of ion-matched waterflooding for EOR.
| Result | Primary mechanisms (direct impact) | Secondary mechanisms (impact by affecting primary mechanism) | Working approaches | Research methods |
|---|---|---|---|---|
| Oil recovery improved | Improving wettability; mineral/ chemical particle/microemulsion movement; oil-water IFT drop/flow characteristics changing; rock surface/pore structure improvement; physical properties improving | I: exchange of multiple ions | (1) decrease salinity (or IS) (2) change the con- centration of PDIs | Core flow experiments, oil/water IFT test, XRD, nuclear magnetic resonance (NMR), zeta potential test, contact angle test, chromatographic analysis |
| II: corrosion deepening | ||||
| III: dissolution/recrystallization of slightly soluble or in-soluble salts | ||||
| IV: micro dispersion of oil and water, IFT changes |
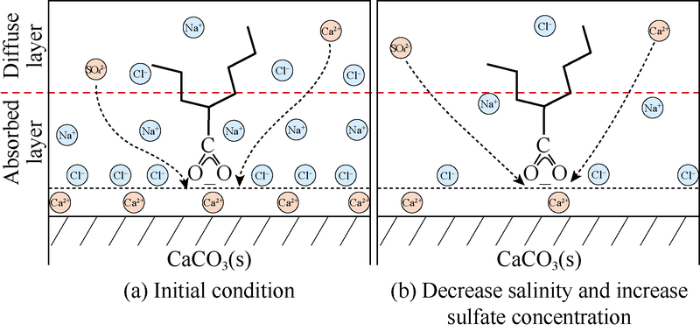
Secondary mechanism I (exchange of multiple ions) mainly involves univalent ions (Na+ and Cl-) and divalent ions (Ca2+, Mg2+ and SO42-) (Fig. 2). Among them, Ca2+ and Mg2+ control the polarity and density of charge, affecting the interaction between crude oil and minerals on the surface of rock[13], thereby affecting the potential of injected water in improving wettability; SO42- acts as a catalyst in the process[14]. It is generally believed that Na+ and Cl- have a weak influence on the oil displacement effect, but they affect the charge density of the absorbed electron layer of the rock and the ability of high valence ions to enter the layer[14]. The difference in the activity of Ca2+ and Mg2+ in PDI is affected by temperature. When the temperature is lower than 70 °C, the interaction between Ca2+ and SO42- (or rock surface) is stronger than that of Mg2+; when the temperature is between 70 °C and 130 °C, both are more active. When the temperature is over 130 °C, the activity of Mg2+ exceeds that of Ca2+[5].
Fig. 2.
Schematic diagram of exchange of multiple ions (modified from reference [15]).
As shown in Fig. 2, in the original reservoir state, the carboxylate radical (—COO-) of the polar components in crude oil adsorbed on the surface of positively charged carbonate rock together with the monovalent ions (Cl- and Na+) with high concentration and high charge density, making most of rock surface neutral or oil-wet. When ion-matched water with lower salinity is injected, the charge density of Cl- and Na+ in the absorbed layer decreases, and Ca2+ and Mg2+ in the diffuse layer compete with the rock surface to adsorb carboxylate radical under the catalyzation of SO42-. The "double-layer" repulsion increases, the adhesion energy decreases, and the water-wet degree increases between rock and oil. Secondary mechanism I can change the charge distribution inside the double layers and the ion composition of an aqueous solution, which affects the secondary mechanisms III and IV. At the same time, the wettability change will affect the interaction extent of water-soluble ions with rock surface, and in turn the secondary mechanism II.
Secondary mechanism Ⅱ (dissolution deepening) involves the secondary dissolution of less soluble/slightly soluble substances. Its effect degree in the oilfield is usually smaller than that in laboratory experiment, but it has great effect on ion-matched waterflooding[16]. No matter what type of carbonate rock, there are a large amount of slightly soluble/insoluble substances (such as calcite and dolomite), which have the following dissolution equilibrium:
Some rocks often contain slightly soluble anhydrite and the equilibrium is as follows:
When the salinity of formation water (FW) decreases or the ion composition changes (which can be influenced by the secondary mechanism I), the change of solubility product of slightly soluble/less soluble minerals lead to the migration of the dissolution equilibrium (usually to the right), release of Ca2+, Mg2+ and SO42- in situ, and wettability improvement at the solution site[17,18]. At the same time, ions released at the dissolution site also exchange with the formation fluid, shifting the dissolution equilibrium (usually to the left), and causing reprecipitation on the rock surface. During this process, the dissolution and recrystallization of the mineral substances will eventually lead to changes in the surface roughness of the rock[12]. In addition, the injected water with low salinity and rich SO42- usually weakens the mechanical properties (such as strength, yield and volume modulus) of Cretaceous limestone, which has a dual impact on reservoirs with low permeability and strong heterogeneity, improving pore-throat connectivity, and reducing the physical parameters[5]. The physical and chemical actions in this mechanism are usually slow (occurring after more than 12 h in laboratory experiments).
Secondary mechanism Ⅲ (dissolution and recrystallization of less soluble and slightly soluble salts) is the result of the combination of secondary mechanisms Ⅰ and Ⅱ, especially the solubility of CaCO3 and CaSO4 are highly susceptible to temperature, salinity and the relative contents of ions in the aqueous solution. If some substances flocculate or precipitate and cause particle migration, other corresponding mechanisms will take control and the seepage law of injected water will be affected correspondingly[18].
Secondary mechanism Ⅳ (formation of oil and water micro-dispersion and change of IFT) mainly involves reservoir fluid. Under reservoir conditions, the presence of low-salinity water and SO42- can help reduce oil viscosity and oil-water IFT, increase interfacial viscoelasticity[19,20], form microemulsion between water and oil[21,22], and thus changing oil-water rheological characteristics to some extent. Micro-dispersion of oil and water, which is influenced by the polar components in crude oil, is also considered as one of the mechanisms[23]. In addition, the presence of the three PDIs can improve the cohesion and connectivity of oil droplets[24]. IFT of oil and water reduction has been widely concerned because it can directly or indirectly affect oil recovery. Besides directly increasing the capillary number in water flooding, IFT reduction can also reduce the adhesion work of rock, which is conducive to the spread of water on the rock surface. In some cases, PDI can significantly reduce the oil-water IFT[25].
For ion-matched waterflooding in carbonate rock reservoir, a variety of relevant mechanisms have been proposed in previous studies based on observed experimental phenomena. The improvement of oil recovery should be the result of the comprehensive effects of the various mechanisms, all of which are related to the ionic behavior of various water-soluble ions.
2. Effect of ionic behavior on EOR mechanisms
2.1. Water chemistry in ion-matched water flooding
Soluble salts in aqueous solution can reduce the solubility of less soluble salts, which is controlled by IS[28]. This physical significance is directly related to the secondary mechanism Ⅱ. Secondly, IS also affects the non-ideal behavior of ions in water. The higher the ion concentration in aqueous solution, the greater the electrostatic interaction between ions will be, so the behavior of ions will be bound and cannot give full play. The influence extent is characterized by the activity coefficient γ (value 0-1.0). Besides water-soluble ions, the behavior of water-soluble molecules will also be affected. When the IS is greater than 0.1 mol/L, the activity coefficient of water-soluble molecules is less than 1.0[27]. Debye-Huckel theory[26] considers the electrostatic action and thermal motion of ions, and gives the relationship between IS and γ (Table 2).
Table 2 Formula of activity coefficient and application range.
| Name | Formula | Range of application |
|---|---|---|
| Guntelberg appro- ximation formula | $\lg {{\gamma }_{i}}=-A{{z}_{i}}^{2}\frac{{{I}_{\text{S}}}^{1/2}}{1+{{I}_{\text{S}}}^{1/2}}$ | IS≤0.1 mol/L |
| Davies formula | $\lg {{\gamma }_{i}}=-A{{z}_{i}}^{2}\left( \frac{{{I}_{\text{S}}}^{1/2}}{1+{{I}_{\text{S}}}^{1/2}}-\text{0}\text{.3}{{I}_{\text{S}}} \right)$ | 0.1 mol/L<IS≤ 0.5 mol/L. |
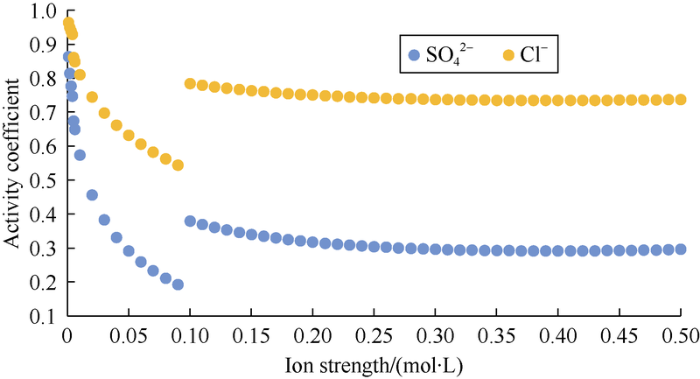
For carbonate rock with positive surface charge, the activity of 2 kinds of anions (Cl- and SO42-) affects their adsorption capacity on the rock surface, and SO42- adsorption is more conducive to improving recovery than Cl- adsorption[5]. The relationships between the activity coefficients of Cl- and SO42- and the IS of solution were plotted by using the formula in Table 2 (Fig. 3).
Fig. 3.
The relationships between activity coefficients and ionic strength of Cl- and SO42-.
It can be seen from Fig. 3 that the activity coefficients of both ions decrease with the increase of IS[26], but the γ decrease of SO42- is greater than that of Cl-. IS can affect the activity of different ions through this difference. From equation (4), IS can be considered as a comprehensive index that takes into account the total salinity, different ion charges and ion concentration at the same time, which is of practical significance in the study of ion-matched waterflooding in carbonate reservoir.
It should be noted that the activity coefficient is only related to the IS of the solution and not directly related to the salinity of the solution. In 1936, W.F.Langelier[27] proposed that the linear correlation between the IS and the salinity was relatively high only when the salinity was less than 1000 mg/L. At present, most researches still believe that the low salinity water reduce the salinity of formation water, which leading to the decrease of oil-water IFT, the generation of micro-dispersion[23], the improvement of wettability[17] and the better dissolution of slightly and less soluble substances[16,25], thus changing the original residual oil state at last[29]. The above mechanisms can be understood as the reduction of IS affecting the activity degree of different ions, and then affecting the various mechanisms in Table 1, which is the essential reason for low-salinity water flooding to improve recovery.
2.2. Differences in the effects of ions on ion strength
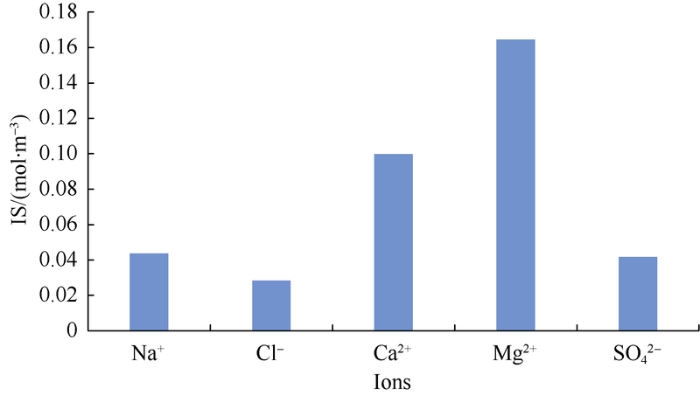
Results of many studies currently show that the SO42- content has critical influence on various mechanisms[30]. Fig. 4 shows the column diagram of the relationship between water-soluble ions and IS at the same mass concentration (1000 mg/L). It can be seen that different ions have different influences on IS, in which Mg2+ has the maximal and Cl- minimal influence. It means that among the three ions of PDI, increasing the concentration of SO42- is more conducive to maintain the IS and thus maintaining the activity of ions. LIN Meiqin et al.[31] proposed in 2018 that the carbonate reservoir was suitable for high-salinity seawater or high-salinity NaSO4 water flooding from the perspective of interaction between ions and molecules. The injection water in their study was also a low IS water rich in SO42-. In addition to SO42-, how to control the Ca2+ and Mg2+ which are PDIs but have a great influence on IS? We think their dual effects on absolute concentration and IS of solution should be considered.
Fig. 4.
Effect of different water-soluble ions on ionic strength.
3. Ion-matched water flooding in Mishrif limestone reservoir of Halfaya oilfield
3.1. Adaptability of ion-matched water flooding to reservoir conditions of Mishrif Formation
The Mishrif Formation in Halfaya oilfield is composed of bioclastic limestone, mainly granular limestone[32]. Depositing in a clean marine environment, the reservoir has hardly clay minerals[33]. From the perspective of mineralogy, the mechanism of enhanced oil recovery by ion-matched water flooding in this kind of carbonate reservoir is quite different from that in sandstone reservoir. Different minerals different in specific surface area, particle structure and crystal structure have different reactivity to different ions. The Mishrif Formation limestone has better response to water with low IS and high SO42- concentration than dolomite, pure calcite and typical limestone, and its wettability is more easily improved[34]. In this study, 3 pieces of natural core of Mishrif reservoir were selected randomly for XRD test. The results are shown in Table 3.
Table 3 Mineral composition of the Mishrif natural core.
| Number | Main mineral content/% | |||
|---|---|---|---|---|
| Quartz | Potassium feldspar | Calcite | Dolomite | |
| 1 | 0.6 | 0.4 | 97.2 | 1.8 |
| 2 | 0.5 | 0.3 | 94.6 | 4.6 |
| 3 | 0.5 | 0.3 | 96.2 | 2.9 |
Generally speaking, the higher the calcite content and the lower the dolomite content in the rock, the greater the decrease of contact angle after the same low salinity waterflooding and the more water-wet the rock will be[17,35]. Therefore, it can be seen from Table 3 that the reservoir has petrological conditions for ion-matched water flooding.
The heterogeneity of the carbonate reservoir in Mishirif is mainly affected by the development of secondary pores (dissolution pores and moldic pores, etc.). In addition, the formation water has a high content of some major mineral ions due to the complex transformation of fluid dissolution and other processes in the later stage of sedimentation. The ion composition of the formation water reflects the original ionic equilibrium state of the carbonate reservoir to some extent, and affects the potential of the injected water to enhance oil recovery[29].
It can be seen from Table 4 that the formation water in Mishrif carbonate formation is CaCl2 type with high salinity and IS. From Table 2, when IS>0.5 mol/L, there is no definite formula to calculate the corresponding activity coefficient. Therefore, according to a specific case (IS=0.7 mol/L, the activity coefficient of SO42- is 0.11, and the activity coefficient of Cl- is 0.68)[27], under the Mishrif Formation water condition (IS≥3.5 mol/L), the activity coefficient of PDIs is less than 0.11, and the difference between the activity coefficients of SO42- and Cl- is greater than 0.57, moreover, the concentration of catalytic ion SO42- is low, so the variety of mechanisms are in an inefficient equilibrium.
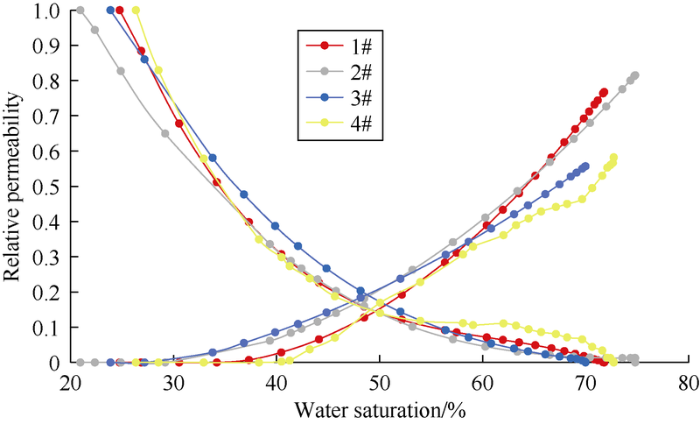
Four cores were selected for relative permeability tests, and the results are shown in Fig. 5, which indicate that the reservoir is inclined to oil-wet (saturation with equal relative permeability is less than 50%), and the relative permeability of water under residual oil saturation is higher (greater than 0.5). Different types of cores (with different initial degrees of dissolution) have different seepage patterns.
The temperature of the reservoir and the composition of the crude oil can also affect the effectiveness of the ion-matched water flooding. The Mishrif reservoir oil has contents of asphaltene, colloid, aromatic hydrocarbon and saturated hydrocarbon of 1.24%, 5.41%, 24.2% and 69.15%, respectively, and a total acid value of 0.24 mg/g, which meet the basic conditions for ion-matched water flooding[18,36-37]. The reservoir temperature is 90 °C, also favorable for such treatment[5].
According to the above analysis on reservoir conditions, the key to ion-matched waterflooding is to reduce the IS of the formation water below a certain value to increase the activity coefficient of PDI and ensure that the concentration of these ions is not too low at the same time.
Fig. 5.
Relative permeability curves of natural core samples from Mishrif Formation reservoir.
3.2. Research approaches
3.2.1. Oil-water interfacial tension tests
In view of the direct and indirect influence of oil-water IFT on oil displacement efficiency, the current water source (Table 4) was treated according to Table 5 to evaluate the IFT under three conditions, to examine the influence of salinity, IS and PDI on IFT: (1) same ion composition but different salinity; (2) same salinity but different ion composition; (3) different absolute concentrations of PDIs.
Table 4 Ion composition of injection water and water source of Mishrif carbonate formation in Halfaya oilfield.
| Water source | Na+/(mg•L-1) | K+/(mg•L-1) | Ca2+/(mg•L-1) | Mg2+/(mg•L-1) | Cl-/(mg•L-1) | SO42-/(mg•L-1) | TDS/(mg•L-1) | IS/(mol•L-1) |
|---|---|---|---|---|---|---|---|---|
| River water | 260 | 4 | 151 | 70 | 390 | 527 | 1573 | 0.04 |
| Seawater | 14 071 | 0 | 880 | 1 391 | 2 4377 | 3 217 | 43 959 | 0.88 |
| Formation water | 60 369 | 1 707 | 8 000 | 1 944 | 114 488 | 360 | 18 7 817 | 3.53 |
Table 5 Treatment methods of different water sources.
| Water source | Method |
|---|---|
| Formation water | Dilute 10,20...60 times |
| Seawater | Dilute 2,4...10 times, |
| River water | Dilute 2 times, IS=0.02 mol/L |
| Add NaSO4 to IS=0.05 mol/L, salinity is about 2500 mg/L at this point, | |
| Add NaSO4 and CaCl2 to IS=0.09 mol/L, salinity is about 3000 mg/L at this point |
3.2.2. Core-flooding experiment
Considering the heterogeneity of carbonate rock and the complexity of ion-matched waterflooding mechanism, slug flooding comparison experiments on core (core parameters are shown in Table 6 and scheme design is shown in Table 7) were conducted in the laboratory to test the effect of ion- matched water comprehensively[8]. Two kinds of optimized ion water were obtained by slug comparison experiments, and the best IS and total salinity (TDS) ranges were obtained too. The best injection water was obtained through the followed experiment of the two kinds of water obtained above. The ion composition and ionic behavior of the solutions during the whole process of the experiment were analyzed to reveal the mechanisms.
Table 6 Basic data of the natural core sample from the Mishrif reservoir in Halfaya oilfield.
| Research stage | No. of schemes | Diameter/cm | Length/cm | Porosity/% | Initial oil saturation/% | Permeability/10-3 μm2 |
|---|---|---|---|---|---|---|
| Optimum ranges of TDS and IS | 1# | 3.8 | 6.5 | 14.0 | 73.7 | 12.2 |
| 2# | 3.8 | 6.5 | 21.1 | 79.7 | 33.7 | |
| Preferred water verification | 3# | 3.8 | 6.0 | 7.1 | 72.9 | 30.1 |
Note: In order to reduce the capillary end effect in the short core experiment, the injection rate was relatively fast in the experiment, and the experimental results are only used for qualitative comparison.
Table 7 Research scheme of slug comparison.
| Research stage | No. of schemes | Water source | Injection schemes | ||||
|---|---|---|---|---|---|---|---|
| Slug 1 | Slug 2 | Slug 3 | Slug 4 | Slug 5 | |||
| Optimum ranges of TDS and IS | 1# | Formation water | Formation water | 10 times dilution | 20 times dilution | 30 times dilution | / |
| 2# | Seawater | Seawater | 2 times dilution | 4 times dilution | 6 times dilution | 8 times dilution | |
| Verification of preferred water | 3# | Formation water and seawater | Optimal dilution times of formation water | Optimal dilution times of seawater | / | / | |
The experimental oil was a mixture of crude oil and aviation kerosene, with a viscosity of 2.5 mPa·s at 90 °C. The experiment was designed at a displacement rate of 0.6 mL/min and carried out according to the following procedures. (1) The core was saturated with formation water and simulation oil; (2) the core was aged for 3 w at 90 °C; (3) the core was displaced with different slugs in sequence according to the scheme in Table 7, the next slug was injected (displacing without interruption) when the water cut of one slug displacement was greater than 99% for 30 min, and so on.
In addition, the following measures were taken to improve the experiment quality: (1) Considering the core size and measurement accuracy, the increment in oil displacement efficiency of less than 1% was not regarded as effective improvement; (2) no precipitation was formed in the injection water, the focus was to study the microscopic oil displacement mechanism, with the effect of secondary mechanism III on sweep efficiency excluded.
4. Results and discussion
4.1. Evaluation of injected water
4.1.1. Extended formula of ion activity coefficient
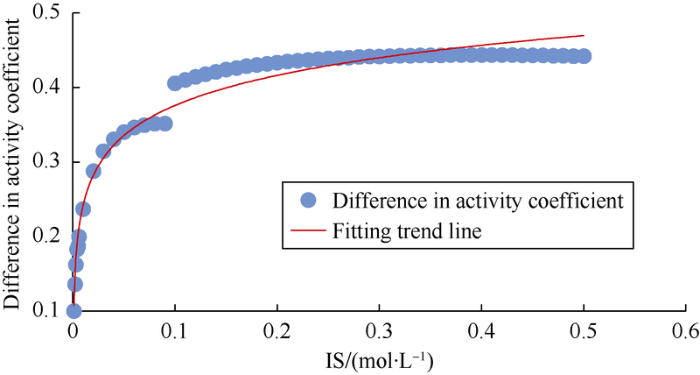
Except river water, the rest water used in this study have higher IS (Table 4), so the formula in Table 2 is not applicable. When IS is more than 0.5 mol/L, there is no accurate formula to calculate the corresponding relationship of IS and activity coefficient. According to the formula in Table 2, the activity coefficient difference curve of SO42- and Cl- was plotted (Fig. 6), and the empirical formula was fitted accordingly.
Equation (5) has a high correlation coefficient (R2=0.969 8) and is only used for approximate characterization in the case of IS>0.5 mol/L.
Fig. 6.
Activity coefficient difference curve of SO42- and Cl-.
4.1.2. Evaluation parameters of ion effective concentration and injected water properties
The inefficient equilibrium state of the initial Mishrif carbonate reservoir (IS>3.0 mol/L) is influenced by the high absolute concentration of monovalent ions and the large difference in the activity coefficients of SO42- and Cl-. In this study, the effective concentration cv was defined to characterize the combined action of ion concentration and activity coefficient.
The higher the effective concentration of the ion, the more the number of real active ions and the stronger the behavior of the ion. The effective concentration will directly affect the secondary mechanisms I, II and indirectly influence the secondary mechanisms III, IV, and is closely related to enhanced oil recovery.
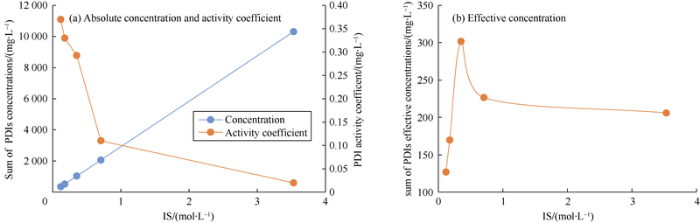
To comprehensively consider the effects of effective concentration of the three PDIs on various mechanisms and oil displacement efficiency, the sum concentration of the three PDIs was used for characterization. At the same time, equation (6) was used to calculate the relationship between the sum of the PDIs concentration, activity coefficient, and the sum of effective concentration with the change of IS in the case that the IS of formation water keeps decreasing under the condition of continuous dilution (Fig. 7).
Fig. 7.
The relationships between the sum of concentration, the sum of effective concentration and the activity coefficient of PDIs in formation water with IS.
As shown in Fig. 7a, the activity coefficient and the concentration of PDIs have opposite correlations with IS of a solution, indicating that there is an optimal range of IS which makes the effective concentration value of PDI maximize (Fig. 7b). Within the optimal IS range, the weights of the influences of ion concentration and activity coefficient on the effective concentration of PDI transform. In other words, when the IS is lower, the injection water mainly increase the PDI concentration, otherwise, it mainly increases the activity coefficient. Here, one (or more) "parameters" related to the effective concentration can be defined. When the "parameter" of the latter slug is lower than that of the former slug, there will be little improvement in oil displacement efficiency further. The parameter was used to compare slug schemes with different IS or TDS values designed in Table 7. Therefore, in addition to the TDS, “three parameters” related to IS and effective concentration were established for further study. They are (1) SO42- effective concentration, (2) sum of effective PDIs concentration, and (3) difference between the effective concentrations of SO42- and Cl-.
4.2. Relationship between the mechanism of oil-water interfacial tension reduction and effective ion concentration
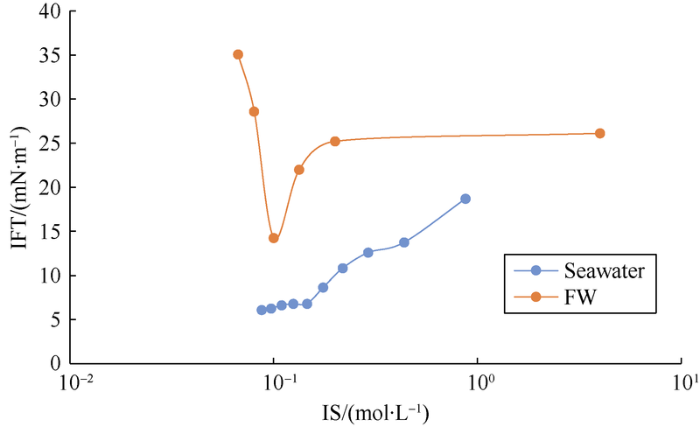
Previous studies have shown that diluting aqueous solution in proportion will lead to the decline of IS, and then the drop of absolute concentrations and the rise of activity coefficients of all ions in the water (especially high valence ions). Fig. 8 shows that the IFT between crude oil and seawater with a higher concentration of SO42- is lower than that of formation water lacking SO42- under the same IS. In addition, they show the following differences with the change of IS. The IFT between seawater and crude oil basically decreases with the decrease of IS, and the decrease of SO42- concentration does not change the decline trend of IFT, so the main way to optimize seawater is to increase the activity coefficient of SO42-. In contrast, the IFT between formation water and crude oil has a lowest (good) value, and the change ranges of SO42- concentration and activity coefficient affect the decreasing trend of IFT. Therefore, the main way of formation water optimization is to increase both the ion concentration and activity coefficient of SO42-. This rule reflects the weight transformation of the influence of the concentration and activity coefficient of SO42- on the IFT reduction. In addition, the optimal IS of the two kinds of water within the test range are both approximately 0.1 mol/L.
Fig. 8.
The variation of oil-water interfacial tension with IS.
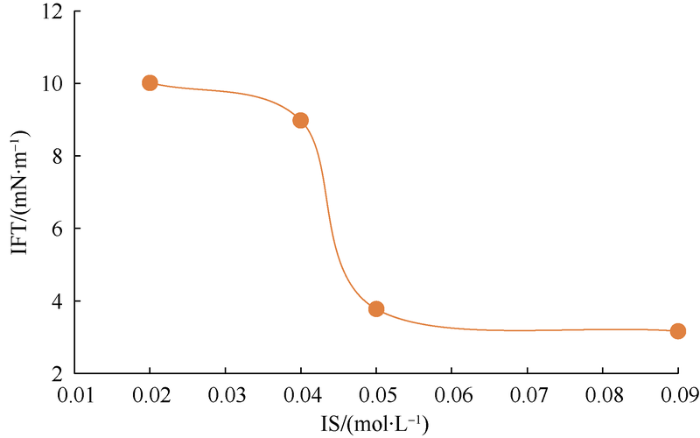
For river water with lower IS, according to the dilution scheme of the formation water in Table 5 (Fig. 9), the test results show that when IS was less than 0.1 mol/L, the IFT decreased with the increase of IS. After the addition of SO42- (IS value increased from 0.04 mol/L to 0.05 mol/L), the IFT decreased significantly. After the addition of Ca2+ (IS value increased from 0.05 mol/L to 0.09 mol/L), the IFT basically unchanged. The IFT tests of the water samples from 3 sources show that the effective concentration of SO42- is closely related to the IFT mechanism.
Fig. 9.
The variation of interfacial tension between oil and river water samples treated by different ways with IS.
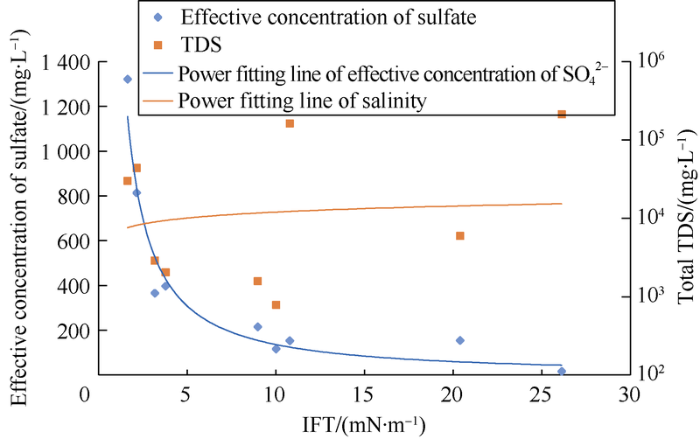
At present, there are 9 kinds of water sources composed of different ions (including optimized water) in the Halfaya oilfield area. The oil-water IFT, TDS, effective concentration and other parameters were plotted and curve fitted (Fig. 10). It can be found that IFT is better correlated with the effective concentration of SO42- .
Fig. 10.
Fitting relationship of oil-water interfacial tension, TDS and effective concentration.
For sandstone, the critical capillary number is very large, so surfactant flooding can improve recovery efficiency only when IFT is ultra-low (orders of 1×10-3 mN/m). Currently, ion-matched water can only reduce the IFT to the order of 1×100 mN/m, and the capillary number is relatively small, so the role of this mechanism can be neglected. However, in carbonate reservoir, according to the extending study of classic capillary desaturation curve (CDC)[38], the crude oil changes from wetting phase to non-wetting phase and the pores are wide in size range, the critical capillary number required to improve oil displacement efficiency will significantly reduce to about 1×10-7. The capillary number under the experimental conditions in this study calculated by the capillary number formula was up to 1×10-6 (higher than the critical capillary number). At present, a research on carbonate rock showed that when the oil-water IFT decreased from 40 mN/m to 3 mN/m, the oil displacement efficiency increased by 4%[39]. This means that the drop of IFT for enhancing oil recovery cannot be ignored.
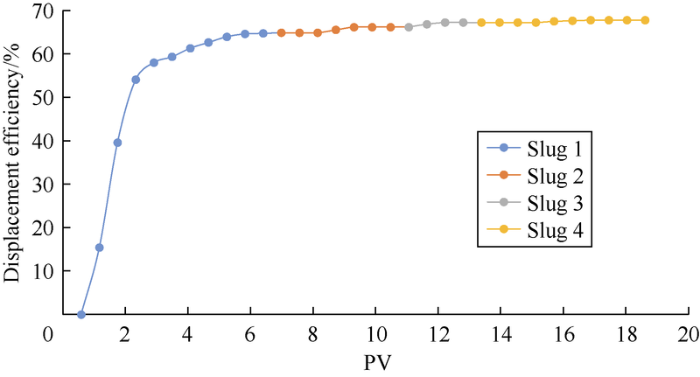
Fig. 11.
Displacement efficiency curve of experiment 1#.
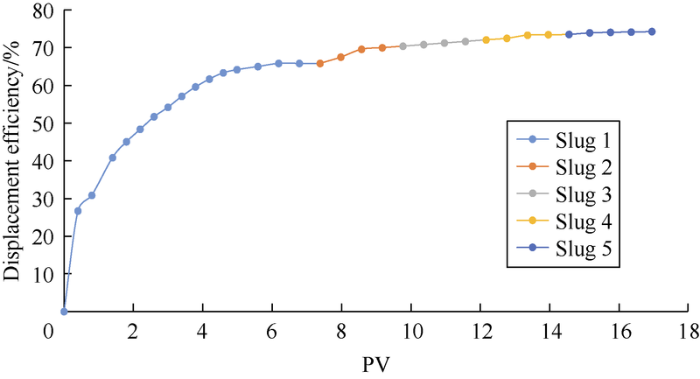
Fig. 12.
Displacement efficiency curve of experiment 2#.
4.3. The best range of salinity and IS of ion-matched water
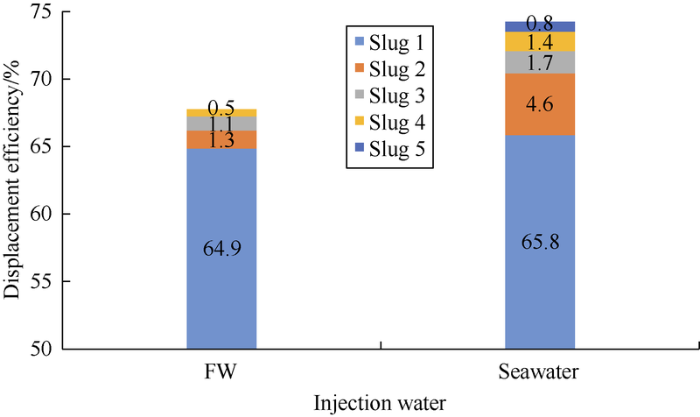
Fig. 11 and Fig. 12 show the oil displacement effect curves of schemes 1# and 2#. It can be seen that diluted ion-matched water can improve oil displacement efficiency, among which the second slug has the most obvious effect. The oil-increasing effect of each slug was quantified to make comparison (Fig. 13). The formation water slug 3 and the seawater slug 4 are critical slugs (the next slug improves oil displacement efficiency by less than 1%). Formation water slug 2 increased oil displacement efficiency by 1.3%, and the 2 formation water slugs with different dilution ratios increased oil displacement efficiency by 2.4% combined (excluding the slug with oil displacement efficiency increase of less than 1%). The second slug of seawater increased oil displacement efficiency by 4.6%, and the 3 seawater slugs with different dilution ratio increased oil displacement efficiency by 7.7%. The TDS and IS intervals of the critical slug and the next slug are taken as the optimal range (Table 8). When IS is less than 0.11 mol/L, reducing IS can’t further effectively improve oil displacement efficiency. Among them, the optimal IS range of formation water is close to that predicted by the sum of the effective concentrations of PDIs in the “three parameters” (less than 0.50 mol/L) (Fig. 7). The difference between the two may be due to the fact that the sum of the effective PDI concentrations is a simple sum of the concentrations of the three kinds of PDI ions, so the catalytic effect of SO42- and the difference between different ions cannot be distinguished. The influences of concentrations of the PDI ions on recovery efficiency should have a more complex functional relationship. Moreover, the optimal IS ranges of the two kinds of water were slightly larger than the IFT test results. According to theoretical analysis, IFT tests and core-flooding experiments, it is suggested that the injection water for the Mishrif carbonate reservoir have a salinity of 5500-10 000 mg/L and IS of 0.11-0.18 mol/L under current conditions.
Fig. 13.
Comparison of oil displacement efficiency of slugs in experiments 1# and 2#.
Table 8 The optimum salinity and IS ranges of formation water and seawater.
| Water type | TDS/(mg•L-1) | IS/(mol•L-1) |
|---|---|---|
| Formation water | 6261-9391 | 0.18-0.12 |
| Seawater | 5495-7327 | 0.15-0.11 |
At present, studies on the optimal range of salinity of injection water suggest that the EOR mechanism of ion-matched water flooding in sandstone is simpler than that in carbonate rock, and the best range of salinity is generally between 2000 mg/L and 7000 mg/L[40]. However, in the carbonate reservoir, it has been proved that the recovery factor can be effectively improved when the salinity of injection water is between 20 000 and 33 000 mg/L[11] or between 5000 and 10 000 mg/L[10]. The optimal salinity range of injection water in carbonate reservoir is more difficult to determine, because the recovery improvement is a process jointly affected by multiple mechanisms directly or indirectly. Neither ion concentration or ion behavior characterization based on total salinity parameter nor ion matching water evaluation and selection based on single mechanism is reliable for carbonate reservoirs.
Table 9 Main ion composition of two kinds of optimized water.
| Type of water | Number | Na+/ (mg•L-1) | K+/ (mg•L-1) | Ca2+/ (mg•L-1) | Mg2+/ (mg•L-1) | Cl-/ (mg•L-1) | SO42-/ (mg•L-1) | TDS/ (mg•L-1) | IS/ (mol•L-1) |
|---|---|---|---|---|---|---|---|---|---|
| Formation water diluted 20 times | 1 | 2 352 | 85.4 | 400.0 | 97.2 | 5 000.0 | 18.0 | 8 000 | 0.15 |
| Seawater diluted 6 times | 2 | 2 750 | 0 | 146.7 | 231.8 | 4 331.4 | 536.2 | 8 000 | 0.16 |
Note: matching the water to the same salinity of 8 000 mg/L with NaCl
4.4. Comparison of 2 kinds of ion-matched water and "3 parameters" validation
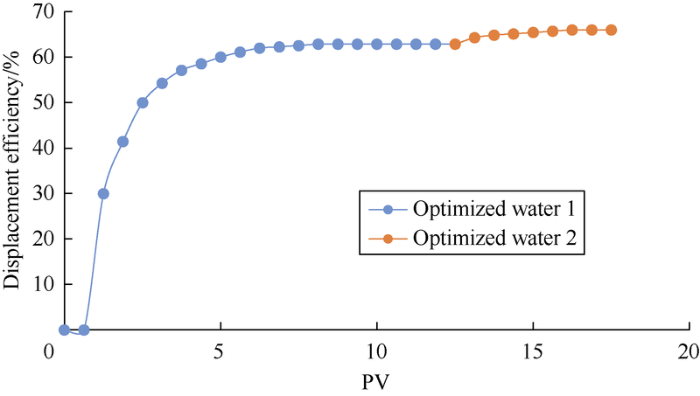
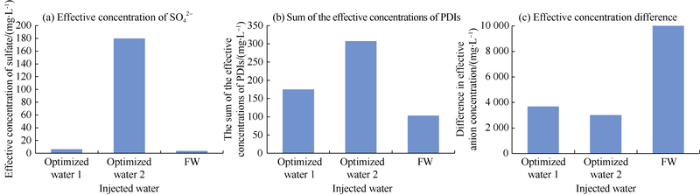
Table 9 shows the main ion composition of the two kinds of optimized ion-matched water, it can be seen their salinities and IS are both in the optimal ranges. At the same total salinity, the optimized water 2 with higher IS has an oil displacement efficiency of about 3.14% higher than the optimized water 1 (Fig. 14). By calculating and comparing the "three parameters" of the two kinds of optimized water and original formation water (Fig. 15), two rules can be found: (1) The “three parameters” of the two kinds of optimized water are superior to formation water (although the absolute concentrations of Ca2+ and Mg2+ in formation water are higher). (2) The "three parameters" of optimized water 2 are better than that of optimized water 1, especially the difference in the effective concentration of anions is large. Therefore, after contacting with the formation water, the injected ion-matched water can break the original physical-chemical equilibrium in the reservoir, and form a new equilibrium which is more conducive to oil displacement to increase oil recovery. The "three parameters" of the injection water have a greater influence on the oil displacement efficiency than the salinity and ion concentration.
Fig. 14.
Displacement curves of two kinds of optimized ion-matched water.
The modeling experiment in lab and numerical simulation currently cannot fully simulate the complex conditions of carbonate reservoir and the dynamic changes of various influencing factors. Therefore, the current understandings on the EOR mechanisms of ion-matched water flooding are still controversial. For Mishrif limestone reservoir in Halfaya oilfield, higher oil displacement efficiency can be achieved by making the injection water meet the IS (or TDS) range shown in Table 8. Seawater diluted 6 times is the ideal injection water for the reservoir.
In addition to diluting the water, ion-matching technology can also accurately design the ion composition of the injection water according to specific formation conditions and injection stages. For example, the formation water in Table 4 has higher concentrations of Ca2+ and Mg2+, and after reducing the IS for decreasing the concentration of Ca2+ and Mg2+, the two kinds of ions are supplemented in-situ by the influence of the IS and the secondary mechanism II. Therefore, in the early stage of water injection, increasing the concentration of SO42- may achieve effective recovery improvement. However, when the injected PV is large, little original formation water is left or in a bound state, and new ion equilibrium is formed between the rock and injection water, the concentration of Ca2+ and Mg2+ may need to be appropriately increased while maintaining the range of IS. This requires further study.
Fig. 15.
"Three parameters" of injection water and original formation water.
5. Conclusions
The mechanisms of EOR by ion-matched water-flooding in carbonate reservoirs are: (1) reducing the ion strength of formation water and matching the ion composition of formation water, so as to reduce the effective concentration difference between monovalent and divalent ions on the surface of carbonate rock, and increase the effective concentration of PDI (especially SO42-); (2) improving wettability, oil-water interface properties, and the pore structure and physical properties of the reservoir slightly, finally, while breaking the original formation ion equilibrium, establishing a new equilibrium conducive to water-flooding to improve the oil displacement effect.
Compared with salinity, ion strength is more suitable to characterize the effect of ion behavior on the mechanisms of ion-matched waterflooding in carbonate reservoirs: According to the ion strength, the effective concentration can be determined, and then the "three parameters" (the effective concentration of SO42-, the sum of effective concentrations of PDI ions, the difference between the effective concentration of SO42- and Cl-) can be worked out to effectively characterize the close relationship between the ion behavior and the oil displacement mechanisms.
When ion-matched waterflooding is carried out in the Mishrif carbonate reservoir in Halfaya oilfield, the injected water has an optimal range of salinity of 5500 to 10 000 mg/L and optimal range of ion strength from 0.11 to 0.18 mol/L. Seawater diluted 6 times is more suitable for injection in this reservoir. Compared with ordinary seawater, this kind of water can increase oil displacement efficiency by more than 4.6%. Compared with the formation water with the best dilution ratio, it can increase oil displacement efficiency by 3.14%.
Nomenclature
A—coefficient related to the dielectric constant of water, when the temperature is 25 °C, it equals 0.5, and increases slightly with the increase of temperature;
c—ion concentration, mg/L;
cV—effective concentration of ion, mg/L;
i—ion number;
n—the total number of ions;
IS—ion strength, mol/L;
z—charge on ions;
M—molarity of ion, mol/L;
γ—activity coefficient, dimensionless.
Reference
Theories and practices of carbonate reservoirs development in China
NMR study of carbonates wettability
DOI:10.1016/j.petrol.2017.06.023 URL [Cited within: 1]
Pore types, origins and control on reservoir heterogeneity of carbonate rocks in Middle Cretaceous Mishrif Formation of the West Qurna oilfield, Iraq
DOI:10.1016/j.petrol.2018.08.034 URL [Cited within: 1]
Experimental evaluation of carbonated brine-limestone interactions under reservoir conditions: Emphasis on the effect of core scale heterogeneities
DOI:10.1016/j.ijggc.2017.11.002 URL [Cited within: 1]
Brine-dependent recovery processes in carbonate and sandstone petroleum reservoirs: Review of laboratory-field studies, interfacial mechanisms and modeling attempts
DOI:10.3390/en11010001 URL [Cited within: 6]
Quantification of wettability characteristics for carbonates using different salinities
DOI:10.1016/j.petrol.2018.10.044 URL [Cited within: 1]
Advanced water flooding in chalk reservoirs: Understanding of underlying mechanisms
DOI:10.1016/j.colsurfb.2019.110711
URL
PMID:31864114
[Cited within: 1]

Encapsulation into nanocarriers, such as niosomes, is a promising way to protect them from degradation, and allow controll and target delivery of bioactive compounds. For biotechnological applications, a tight control of particle size with acceptable encapsulation efficiencies (EE) is a technological challenge, especially for hydrophilic compounds due to its capability to diffuse across biological barriers. Niosomes formulated with mixture of surfactants represent promising nanocarriers due to the advantages of non-ionic surfactants, such as low cost, versatility and enhanced physico-chemical properties. In this work, the effect of both, composition of the hydrating solution and molecular weight of the loaded compound, on the particle size and EE of niosomes prepared by using the thin film hydration method was studied. Particularly, mili-Q water, glycerol solution and PEG-400 solution were tested for niosomes formulated with Span®80-Tween®80 with/without dodecanol as membrane stabilizer. It was found that particle size highly depends on hydration media composition and an interaction with compound MW could exist. Larger vesicles results in an increase in EE, which could be purely related with physical aspects such as vesicle loading volume capacity. The effect of hydration solution composition could be related with their ability to change the bilayer packing and physical properties, as observed by differential scanning calorimetry. Finally, it was possible to compare the suitability of dialysis and gel filtration as purification methods, demonstrating that gel filtration is not an adequate purification method when viscous solutions are used, since they could affect the particle vesicles retention and hence EE measurements would be misrepresentative.
Waterflooding in carbonate reservoirs: Does the salinity matter?
DOI:10.1631/jzus.B1700586
URL
PMID:30614230
[Cited within: 2]

Globally, peptide-based anticancer therapies have drawn much attention. Marine organisms are a reservoir of anticancer peptides that await discovery. In this study, we aimed to identify cytotoxic oligopeptides from Sarcophyton glaucum. Peptides were purified from among the S. glaucum hydrolysates produced by alcalase, chymotrypsin, papain, and trypsin, guided by a methylthiazolyldiphenyl-tetrazolium bromide (MTT) assay on the human cervical cancer (HeLa) cell line for cytotoxicity evaluation. Purification techniques adopted were membrane ultrafiltration, gel filtration chromatography, solid phase extraction (SPE), and reversed-phase high-performance liquid chromatography (RP-HPLC). Purified peptides were identified by de novo peptide sequencing. From papain hydrolysate, three peptide sequences were identified: AGAPGG, AERQ, and RDTQ (428.45, 502.53, and 518.53 Da, respectively). Peptides synthesized from these sequences exhibited cytotoxicity on HeLa cells with median effect concentration (EC50) values of 8.6, 4.9, and 5.6 mmol/L, respectively, up to 5.8-fold stronger than the anticancer drug 5-fluorouracil. When tested at their respective EC50, AGAPGG, AERQ, and RDTQ showed only 16%, 25%, and 11% cytotoxicity to non-cancerous Hek293 cells, respectively. In conclusion, AERQ, AGAPGG, and RDTQ are promising candidates for future development as peptide-based anticancer drugs.
An experimental investigation into the impact of sulfate ions in smart water to improve oil recovery in carbonate reservoirs
DOI:10.1007/s11242-015-0616-4 URL [Cited within: 1]
Conditions for a low-salinity enhanced oil recovery (EOR) effect in carbonate oil reservoirs
DOI:10.1016/j.saa.2019.117936
URL
PMID:31864151
[Cited within: 2]

TiO2 nanoparticles as solar cells and photocatalysts caused extensive attention in solar energy utilization and environment remediation due to the high photoelectrochemical performance. We demonstrated a novel approach to fabricate big-leaf hydrangea-like Bi2S3-BiOBr self-assembled by superthin nanosheets on TiO2 nanotube arrays (TiO2 NTs/B2S3-BiOBr). Results indicated that the Bi2S3-BiOBr co-sensitization showed higher photoelectric conversion efficiency than the single Bi2S3 or BiOBr sensitization. More remarkably, TiO2 NTs/B2S3-BiOBr showed excellent photoelectrocatalytic (PEC) removal of MB, MO, RhB and Cr(VI). The remarkable PEC performance could be attributed to the strong visible light absorption and effective electron transportation at the interface of TiO2/B2S3-BiOBr. The high photoelectrochemical performances indicate that the TiO2 NTs/B2S3-BiOBr could work as potential photoelectric materials for large-scale applications in the photoelectrochemical energy conversion and pollutant removal.
Smart water as wettability modifier in carbonate and sandstone: A discussion of similarities/differences in the chemical mechanisms
DOI:10.1016/j.saa.2019.117936
URL
PMID:31864151
[Cited within: 2]

TiO2 nanoparticles as solar cells and photocatalysts caused extensive attention in solar energy utilization and environment remediation due to the high photoelectrochemical performance. We demonstrated a novel approach to fabricate big-leaf hydrangea-like Bi2S3-BiOBr self-assembled by superthin nanosheets on TiO2 nanotube arrays (TiO2 NTs/B2S3-BiOBr). Results indicated that the Bi2S3-BiOBr co-sensitization showed higher photoelectric conversion efficiency than the single Bi2S3 or BiOBr sensitization. More remarkably, TiO2 NTs/B2S3-BiOBr showed excellent photoelectrocatalytic (PEC) removal of MB, MO, RhB and Cr(VI). The remarkable PEC performance could be attributed to the strong visible light absorption and effective electron transportation at the interface of TiO2/B2S3-BiOBr. The high photoelectrochemical performances indicate that the TiO2 NTs/B2S3-BiOBr could work as potential photoelectric materials for large-scale applications in the photoelectrochemical energy conversion and pollutant removal.
Time-dependent physico-chemical changes of carbonate surfaces from SmartWater (diluted seawater)-flooding processes for improved oil recovery
DOI:10.1021/acs.langmuir.8b02711
URL
PMID:30509072
[Cited within: 2]

Over the past few decades, field- and laboratory-scale studies have shown enhancements in oil recovery when reservoirs, which contain high-salinity formation water (FW), are waterflooded with modified-salinity salt water (widely referred to as the low-salinity, dilution, or SmartWater effect for improved oil recovery). In this study, we investigated the time dependence of the physicochemical processes that occur during diluted seawater (i.e., SmartWater) waterflooding processes of specific relevance to carbonate oil reservoirs. We measured the changes to oil/water/rock wettability, surface roughness, and surface chemical composition during SmartWater flooding using 10-fold-diluted seawater under mimicked oil reservoir conditions with calcite and carbonate reservoir rocks. Distinct effects due to SmartWater flooding were observed and found to occur on two different timescales: (1) a rapid (<15 min) increase in the colloidal electrostatic double-layer repulsion between the rock and oil across the SmartWater, leading to a decreased oil/water/rock adhesion energy and thus increased water wetness and (2) slower (>12 h to complete) physicochemical changes of the calcite and carbonate reservoir rock surfaces, including surface roughening via the dissolution of rock and the reprecipitation of dissolved carbonate species after exchanging key ions (Ca2+, Mg2+, CO32-, and SO42- in carbonates) with those in the flooding SmartWater. Our experiments using crude oil from a carbonate reservoir reveal that these reservoir rock surfaces are covered with organic-ionic preadsorbed films (ad-layers), which the SmartWater removes (detaches) as flakes. Removal of the organic-ionic ad-layers by SmartWater flooding enhances oil release from the surfaces, which was found to be critical to increasing the water wetness and significantly improving oil removal from carbonates. Additionally, the increase in water wetness is further enhanced by roughening of the rock surfaces, which decreases the effective contact (interaction) area between the oil and rock interfaces. Furthermore, we found that the rate of these slower physicochemical changes to the carbonate rock surfaces increases with increasing temperature (at least up to an experimental temperature of 75 °C). Our results suggest that the effectiveness of improved oil recovery from SmartWater flooding depends strongly on the formation of the organic-ionic ad-layers. In oil reservoirs where the ad-layer is fully developed and robust, injecting SmartWater would lead to significant removal of the ad-layer and improved oil recovery.
Temperature dependence of the zeta potential in intact natural carbonates
DOI:10.1002/2016GL071151 URL [Cited within: 1]
Wettability alteration in carbonates during “Smart Waterflood”: Underlying mechanisms and the effect of individual ions
DOI:10.1016/j.colsurfa.2015.09.067 URL [Cited within: 2]
Modified seawater as a smart EOR fluid in chalk
DOI:10.1016/j.petrol.2015.06.034 URL
Modeling investigation of low salinity water injection in sandstones and carbonates: Effect of Na + and $SO_{4}^{2-}$
DOI:10.1016/j.fuel.2018.05.161 URL [Cited within: 2]
Insights into the mechanism of wettability alteration by low-salinity flooding (LSF) in carbonates
DOI:10.1021/ef5023847 URL [Cited within: 3]
Interactions of fines with oil and its implication in smart water flooding
DOI:10.1016/j.scitotenv.2019.135978
URL
PMID:31864138
[Cited within: 3]

Scientific evidences abound of the occurrence of plastic pollution, from mega- to nano-sized plastics, in virtually all matrixes of the environment. Apart from the direct effects of plastics and microplastics pollution such as entanglement, inflammation of cells and gut blockage due to ingestion, plastics are also able to act as vectors of various chemical contaminants in the aquatic environment. This paper provides a review of the association of plastic additives with environmental microplastics, how the structure and composition of polymers influence sorption capacities and highlights some of the models that have been employed to interpret experimental data from recent sorption studies. The factors that influence the sorption of chemical contaminants such as the degree of crystallinity, surface weathering, and chemical properties of contaminants. and the implications of chemical sorption by plastics for the marine food web and human health are also discussed. It was however observed that most studies relied on pristine or artificially aged plastics rather than field plastic samples for studies on chemical sorption by plastics.
Interfacial rheological insights of sulfate-enriched smart-water at low and high-salinity in carbonates
DOI:10.1016/j.fuel.2017.06.094 URL [Cited within: 1]
Interfacial viscoelasticity of crude oil/brine: An alternative enhanced-oil-recovery mechanism in smart waterflooding
DOI:10.2118/169127-PA URL [Cited within: 1]
Effects of water soluble ions on interfacial tension (IFT) between oil and brine in smart and carbonated smart water injection process in oil reservoirs
DOI:10.1016/j.molliq.2016.08.089 URL [Cited within: 1]
The impact of monovalent and divalent ions on wettability alteration in oil/low salinity brine/limestone systems
DOI:10.1016/j.molliq.2017.10.095 URL [Cited within: 1]
Fundamental investigation of underlying mechanisms behind improved oil recovery by low salinity water injection in carbonate rocks
DOI:10.1016/j.fuel.2018.01.136 URL [Cited within: 2]
Water ion interactions at crude-oil/water interface and their implications for smart waterflooding in carbonates
DOI:10.2118/183894-PA URL [Cited within: 1]
Investigating injection of low salinity brine in carbonate rock with the assist of works of cohesion and adhesion and spreading coefficient calculations
DOI:10.1016/j.petrol.2017.12.010 URL [Cited within: 2]
4-Water chemistry
DOI:10.1021/acs.chemrev.9b00457
URL
PMID:31804075
[Cited within: 3]

The use of high hydrostatic pressure to investigate structure-function relationships in biomacromolecules in solution provides precise information about conformational changes and variations of the interactions between these macromolecules and the solvent, as well as the volume changes associated with their activity. The complementary use of osmotic pressure reveals quantitatively the extent and direction of the water exchanges between the macromolecules and the solvent and the number of water molecules involved in these exchanges. In this review, the chemistry of ribozymes and the influence of pressure is described. In the case of the hairpin ribozyme, pressure slowed down the self-cleavage reaction on the basis that the formation of the transition state involves a positive ΔV⧧ of activation and the release of 78 ± 4 water molecules. The self-cleaving activity of the hammerhead ribozyme is also slowed down by pressure on the basis of kinetic parameters and ΔVs comparable to those of the hairpin ribozymes. However, it appears that the solution of the hammerhead ribozyme used in this study contains two populations of molecules which differ by the values of these parameters. The results obtained in the case of small self-cleaving ribozymes containing adenine bulges are consistent with the hypothesis that these small RNAs that bind amino acids or peptides could have appeared in prebiotic chemistry under extreme conditions in deep-sea vents or hydrothermal surface sites.
The concept of ionic strength eighty years after its introduction in chemistry
DOI:10.1021/ed081p750 URL [Cited within: 1]
Impact of salinity and connate water on low salinity water injection in secondary and tertiary stages for enhanced oil recovery in carbonate oil reservoirs]
DOI:10.1088/1742-2140/aaae84 URL [Cited within: 2]
Laboratory investigation of the impact of injection-water salinity and ionic content on oil recovery from carbonate reservoirs
DOI:10.1631/jzus.B1700586
URL
PMID:30614230
[Cited within: 1]

Globally, peptide-based anticancer therapies have drawn much attention. Marine organisms are a reservoir of anticancer peptides that await discovery. In this study, we aimed to identify cytotoxic oligopeptides from Sarcophyton glaucum. Peptides were purified from among the S. glaucum hydrolysates produced by alcalase, chymotrypsin, papain, and trypsin, guided by a methylthiazolyldiphenyl-tetrazolium bromide (MTT) assay on the human cervical cancer (HeLa) cell line for cytotoxicity evaluation. Purification techniques adopted were membrane ultrafiltration, gel filtration chromatography, solid phase extraction (SPE), and reversed-phase high-performance liquid chromatography (RP-HPLC). Purified peptides were identified by de novo peptide sequencing. From papain hydrolysate, three peptide sequences were identified: AGAPGG, AERQ, and RDTQ (428.45, 502.53, and 518.53 Da, respectively). Peptides synthesized from these sequences exhibited cytotoxicity on HeLa cells with median effect concentration (EC50) values of 8.6, 4.9, and 5.6 mmol/L, respectively, up to 5.8-fold stronger than the anticancer drug 5-fluorouracil. When tested at their respective EC50, AGAPGG, AERQ, and RDTQ showed only 16%, 25%, and 11% cytotoxicity to non-cancerous Hek293 cells, respectively. In conclusion, AERQ, AGAPGG, and RDTQ are promising candidates for future development as peptide-based anticancer drugs.
Surface wettability control of reservoir rocks by brine
DOI:10.1016/j.cis.2019.03.009
URL
PMID:30999164
[Cited within: 1]

CO2 geo-sequestration is a promising technology to permanently store CO2 in geological formations to control the atmospheric carbon footprint. In addition, CO2 is frequently utilized in enhanced oil recovery operations to accelerate oil production. Both, CO2 geo-storage and EOR, are significantly influenced by the wettability of the associated rock/CO2/brine systems. Wettability drives the multiphase flow dynamics, and microscopic fluid distribution in the reservoir. Furthermore, while wettability is known to be influenced by varying in-situ conditions and surface chemistry of the rock/mineral, the current state-of-the-art indicates wider variabilities of the wetting states. This article, therefore, critically reviews the published datasets on CO2 wettability of geological formations. Essentially, the rock/CO2/brine and rock/crude-oil/CO2-enriched-brine contact angle datasets for the important reservoir rocks (i.e. sandstone and carbonate rocks), as well as for the key minerals quartz and calcite are considered. Also, the parameters that influence wettability are critically analyzed, and the associated parametric trends are discussed and summarized. Finally, we identify pertinent research gaps and define the outlook of future research. The review, therefore, establishes a repository of the recent contact angle data, which thus assists to enhance our current understanding of the subject.
Geological features of grain bank reservoirs and the main controlling factors: A case study on Cretaceous Mishrif Formation, Halfaya Oilfield, Iraq
Optimum development options and strategies on water injection development in carbonate reservoirs in the Middle East
Electrokinetics of carbonate/brine interface in low-salinity waterflooding: Effect of brine salinity, composition, rock type, and pH on zeta-potential and a surface-complexation model
DOI:10.2118/181745-PA URL [Cited within: 1]
Effect of water salinity and rock components on wettability alteration during low-salinity water flooding in carbonate rocks
DOI:10.1007/s12517-018-3611-6 URL [Cited within: 1]
Wettability alteration in chalk: 1. Preparation of core material and oil properties
DOI:10.1016/S0920-4105(00)00083-8 URL [Cited within: 1]
The effect of organic acids on wettability of sandstone and carbonate rocks
DOI:10.1016/j.petrol.2018.01.033 URL [Cited within: 1]
In-situ visualization of multidimensional imbibition in dual-porosity carbonates
DOI:10.2118/170811-PA URL [Cited within: 1]
Evaluation of low-salinity enhanced oil recovery effects in sandstone: Effects of the temperature and pH gradient
DOI:10.1016/j.saa.2019.117936
URL
PMID:31864151
[Cited within: 1]

TiO2 nanoparticles as solar cells and photocatalysts caused extensive attention in solar energy utilization and environment remediation due to the high photoelectrochemical performance. We demonstrated a novel approach to fabricate big-leaf hydrangea-like Bi2S3-BiOBr self-assembled by superthin nanosheets on TiO2 nanotube arrays (TiO2 NTs/B2S3-BiOBr). Results indicated that the Bi2S3-BiOBr co-sensitization showed higher photoelectric conversion efficiency than the single Bi2S3 or BiOBr sensitization. More remarkably, TiO2 NTs/B2S3-BiOBr showed excellent photoelectrocatalytic (PEC) removal of MB, MO, RhB and Cr(VI). The remarkable PEC performance could be attributed to the strong visible light absorption and effective electron transportation at the interface of TiO2/B2S3-BiOBr. The high photoelectrochemical performances indicate that the TiO2 NTs/B2S3-BiOBr could work as potential photoelectric materials for large-scale applications in the photoelectrochemical energy conversion and pollutant removal.